Functional Domains of Autoimmune Regulator (AIRE) Modulate INS-VNTR Transcription in Human Thymic Epithelial Cells
- PMID: 27048654
- PMCID: PMC4900276
- DOI: 10.1074/jbc.M116.722488
Functional Domains of Autoimmune Regulator (AIRE) Modulate INS-VNTR Transcription in Human Thymic Epithelial Cells
Abstract
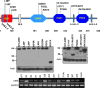
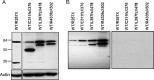
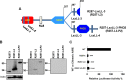
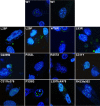
INS-VNTR (insulin-variable number of tandem repeats) and AIRE (autoimmune regulator) have been associated with the modulation of insulin gene expression in thymus, which is essential to induce either insulin tolerance or the development of insulin autoimmunity and type 1 diabetes. We sought to analyze whether each functional domain of AIRE is critical for the activation of INS-VNTR in human thymic epithelial cells. Twelve missense or nonsense mutations in AIRE and two chimeric AIRE constructs were generated. A luciferase reporter assay and a pulldown assay using biotinylated INS-class I VNTR probe were performed to examine the transactivation and binding activities of WT, mutant, and chimeric AIREs on the INS-VNTR promoter. Confocal microscopy analysis was performed for WT or mutant AIRE cellular localization. We found that all of the AIRE mutations resulted in loss of transcriptional activation of INS-VNTR except mutant P252L. Using WT/mutant AIRE heterozygous forms to modulate the INS-VNTR target revealed five mutations (R257X, G228W, C311fsX376, L397fsX478, and R433fsX502) that functioned in a dominant negative fashion. The LXXLL-3 motif is identified for the first time to be essential for DNA binding to INS-VNTR, whereas the intact PHD1, PHD2, LXXLL-3, and LXXLL-4 motifs were important for successful transcriptional activation. AIRE nuclear localization in the human thymic epithelial cell line was disrupted by mutations in the homogenously staining region domain and the R257X mutation in the PHD1 domain. This study supports the notion that AIRE mutation could specifically affect human insulin gene expression in thymic epithelial cells through INS-VNTR and subsequently induce either insulin tolerance or autoimmunity.
Keywords: AIRE; VNTR; gene regulation; insulin; insulin autoimmunity; protein domain; thymus; transcription; type 1 diabetes.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Both polymorphic variable number of tandem repeats and autoimmune regulator modulate differential expression of insulin in human thymic epithelial cells.Diabetes. 2011 Jan;60(1):336-44. doi: 10.2337/db10-0255. Epub 2010 Sep 28. Diabetes. 2011. PMID: 20876716 Free PMC article.
-
Yeast one-hybrid screen of a thymus epithelial library identifies ZBTB7A as a regulator of thymic insulin expression.Mol Immunol. 2013 Dec;56(4):637-42. doi: 10.1016/j.molimm.2013.05.238. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911422 Free PMC article.
-
Functional analysis of SAND mutations in AIRE supports dominant inheritance of the G228W mutation.Hum Mutat. 2005 Oct;26(4):322-31. doi: 10.1002/humu.20224. Hum Mutat. 2005. PMID: 16114041
-
Autoimmune regulator: from loss of function to autoimmunity.Genes Immun. 2003 Jan;4(1):12-21. doi: 10.1038/sj.gene.6363929. Genes Immun. 2003. PMID: 12595897 Review.
-
Aire in Autoimmunity.Annu Rev Immunol. 2024 Jun;42(1):427-53. doi: 10.1146/annurev-immunol-090222-101050. Epub 2024 Jun 14. Annu Rev Immunol. 2024. PMID: 38360547 Free PMC article. Review.
Cited by
-
Aire and Fezf2, two regulators in medullary thymic epithelial cells, control autoimmune diseases by regulating TSAs: Partner or complementer?Front Immunol. 2022 Aug 30;13:948259. doi: 10.3389/fimmu.2022.948259. eCollection 2022. Front Immunol. 2022. PMID: 36110862 Free PMC article. Review.
-
Genetic Mechanisms Highlight Shared Pathways for the Pathogenesis of Polygenic Type 1 Diabetes and Monogenic Autoimmune Diabetes.Curr Diab Rep. 2019 Mar 19;19(5):20. doi: 10.1007/s11892-019-1141-6. Curr Diab Rep. 2019. PMID: 30888520 Free PMC article. Review.
-
Generation and Characterization of iPS Cells Derived from APECED Patients for Gene Correction.Front Endocrinol (Lausanne). 2022 Apr 1;13:794327. doi: 10.3389/fendo.2022.794327. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432216 Free PMC article.
-
Autoimmune Polyglandular Syndrome Type 1: a case report.BMC Med Genet. 2019 Aug 16;20(1):143. doi: 10.1186/s12881-019-0870-3. BMC Med Genet. 2019. PMID: 31420020 Free PMC article.
-
Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea-The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes.Nutrients. 2021 Nov 7;13(11):3972. doi: 10.3390/nu13113972. Nutrients. 2021. PMID: 34836227 Free PMC article. Review.
References
-
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., and Paquette T. L. (1983) Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222, 1337–1339 - PubMed
-
- Kuglin B., Gries F. A., and Kolb H. (1988) Evidence of IgG autoantibodies against human proinsulin in patients with IDDM before insulin treatment. Diabetes 37, 130–132 - PubMed
-
- Ahonen P. (1985) Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED): autosomal recessive inheritance. Clin. Genet. 27, 535–542 - PubMed
-
- Ahonen P., Myllärniemi S., Sipilä I., and Perheentupa J. (1990) Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N. Engl. J. Med. 322, 1829–1836 - PubMed
-
- Finnish-German APECED Consortium (1997) An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains: The Finnish-German APECED Consortium Atuoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat. Genet. 17, 399–403 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

