Maraviroc, as a Switch Option, in HIV-1-infected Individuals With Stable, Well-controlled HIV Replication and R5-tropic Virus on Their First Nucleoside/Nucleotide Reverse Transcriptase Inhibitor Plus Ritonavir-boosted Protease Inhibitor Regimen: Week 48 Results of the Randomized, Multicenter MARCH Study
- PMID: 27048747
- PMCID: PMC5853584
- DOI: 10.1093/cid/ciw207
Maraviroc, as a Switch Option, in HIV-1-infected Individuals With Stable, Well-controlled HIV Replication and R5-tropic Virus on Their First Nucleoside/Nucleotide Reverse Transcriptase Inhibitor Plus Ritonavir-boosted Protease Inhibitor Regimen: Week 48 Results of the Randomized, Multicenter MARCH Study
Abstract
Background: Alternative combination antiretroviral therapies in virologically suppressed human immunodeficiency virus (HIV)-infected patients experiencing side effects and/or at ongoing risk of important comorbidities from current therapy are needed. Maraviroc (MVC), a chemokine receptor 5 antagonist, is a potential alternative component of therapy in those with R5-tropic virus.
Methods: The Maraviroc Switch Study is a randomized, multicenter, 96-week, open-label switch study in HIV type 1-infected adults with R5-tropic virus, virologically suppressed on a ritonavir-boosted protease inhibitor (PI/r) plus double nucleoside/nucleotide reverse transcriptase inhibitor (2 N(t)RTI) backbone. Participants were randomized 1:2:2 to current combination antiretroviral therapy (control), or replacing the protease inhibitor (MVC + 2 N(t)RTI arm) or the nucleoside reverse transcriptase inhibitor backbone (MVC + PI/r arm) with twice-daily MVC. The primary endpoint was the difference (switch minus control) in proportion with plasma viral load (VL) <200 copies/mL at 48 weeks. The switch arms were judged noninferior if the lower limit of the 95% confidence interval (CI) for the difference in the primary endpoint was < -12% in the intention-to-treat (ITT) population.
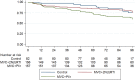
Results: The ITT population comprised 395 participants (control, n = 82; MVC + 2 N(t)RTI, n = 156; MVC + PI/r, n = 157). Baseline characteristics were well matched. At week 48, noninferior rates of virological suppression were observed in those switching away from a PI/r (93.6% [95% CI, -9.0% to 2.2%] and 91.7% [95% CI, -9.6% to 3.8%] with VL <200 and <50 copies/mL, respectively) compared to the control arm (97.6% and 95.1% with VL <200 and <50 copies/mL, respectively). In contrast, MVC + PI/r did not meet noninferiority bounds and was significantly inferior (84.1% [95% CI, -19.8% to -5.8%] and 77.7% [95% CI, -24.9% to -8.4%] with VL <200 and <50 copies/mL, respectively) to the control arm in the ITT analysis.
Conclusions: These data support MVC as a switch option for ritonavir-boosted PIs when partnered with a 2-N(t)RTI backbone, but not as part of N(t)RTI-sparing regimens comprising MVC with PI/r.
Clinical trials registration: NCT01384682.
Keywords: HIV-1; antiretroviral; comorbidity; maraviroc; switch.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
-
- Nakagawa F, Lodwick RK, Smith CJ et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012; 26:335–43. - PubMed
-
- Solomon DA, Sax PE. Current state and limitations of daily oral therapy for treatment. Curr Opin HIV AIDS 2015; 10:219–25. - PubMed
-
- Casado JL, Bañón S, Rodriguez MA et al. Efficacy and pharmacokinetics of the combination of etravirine plus raltegravir as novel dual antiretroviral maintenance regimen in HIV-infected patients. Antiviral Res 2015; 113:103–6. - PubMed
-
- Reliquet V, Chirouze C, Allavena C et al. Nevirapine-raltegravir combination, an NRTI and PI/r sparing regimen, as maintenance antiretroviral therapy in virologically suppressed HIV-1-infected patients. Antivir Ther 2014; 19:117–23. - PubMed
-
- Ofotokun I, Sheth AN, Sanford SE et al. A switch in therapy to a reverse transcriptase inhibitor sparing combination of lopinavir/ritonavir and raltegravir in virologically suppressed HIV-infected patients: a pilot randomized trial to assess efficacy and safety profile: the KITE study. AIDS Res Hum Retroviruses 2012; 28:1196–206. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

