Deacetylation of Fungal Exopolysaccharide Mediates Adhesion and Biofilm Formation
- PMID: 27048799
- PMCID: PMC4817252
- DOI: 10.1128/mBio.00252-16
Deacetylation of Fungal Exopolysaccharide Mediates Adhesion and Biofilm Formation
Abstract
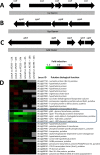
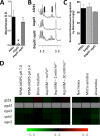
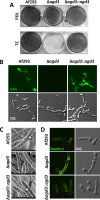
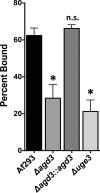
The mold Aspergillus fumigatus causes invasive infection in immunocompromised patients. Recently, galactosaminogalactan (GAG), an exopolysaccharide composed of galactose and N-acetylgalactosamine (GalNAc), was identified as a virulence factor required for biofilm formation. The molecular mechanisms underlying GAG biosynthesis and GAG-mediated biofilm formation were unknown. We identified a cluster of five coregulated genes that were dysregulated in GAG-deficient mutants and whose gene products share functional similarity with proteins that mediate the synthesis of the bacterial biofilm exopolysaccharide poly-(β1-6)-N-acetyl-D-glucosamine (PNAG). Bioinformatic analyses suggested that the GAG cluster gene agd3 encodes a protein containing a deacetylase domain. Because deacetylation of N-acetylglucosamine residues is critical for the function of PNAG, we investigated the role of GAG deacetylation in fungal biofilm formation. Agd3 was found to mediate deacetylation of GalNAc residues within GAG and render the polysaccharide polycationic. As with PNAG, deacetylation is required for the adherence of GAG to hyphae and for biofilm formation. Growth of the Δagd3 mutant in the presence of culture supernatants of the GAG-deficient Δuge3 mutant rescued the biofilm defect of the Δagd3 mutant and restored the adhesive properties of GAG, suggesting that deacetylation is an extracellular process. The GAG biosynthetic gene cluster is present in the genomes of members of the Pezizomycotina subphylum of the Ascomycota including a number of plant-pathogenic fungi and a single basidiomycete species,Trichosporon asahii, likely a result of recent horizontal gene transfer. The current study demonstrates that the production of cationic, deacetylated exopolysaccharides is a strategy used by both fungi and bacteria for biofilm formation.
Importance: This study sheds light on the biosynthetic pathways governing the synthesis of galactosaminogalactan (GAG), which plays a key role in A. fumigatus virulence and biofilm formation. We find that bacteria and fungi use similar strategies to synthesize adhesive biofilm exopolysaccharides. The presence of orthologs of the GAG biosynthetic gene clusters in multiple fungi suggests that this exopolysaccharide may also be important in the virulence of other fungal pathogens. Further, these studies establish a molecular mechanism of adhesion in which GAG interacts via charge-charge interactions to bind to both fungal hyphae and other substrates. Finally, the importance of deacetylation in the synthesis of functional GAG and the extracellular localization of this process suggest that inhibition of deacetylation may be an attractive target for the development of novel antifungal therapies.
Copyright © 2016 Lee et al.
Figures



Similar articles
-
The Transcription Factor SomA Synchronously Regulates Biofilm Formation and Cell Wall Homeostasis in Aspergillus fumigatus.mBio. 2020 Nov 10;11(6):e02329-20. doi: 10.1128/mBio.02329-20. mBio. 2020. PMID: 33173002 Free PMC article.
-
PtaB, a lim-domain binding protein in Aspergillus fumigatus regulates biofilm formation and conidiation through distinct pathways.Cell Microbiol. 2018 Jan;20(1). doi: 10.1111/cmi.12799. Epub 2017 Nov 17. Cell Microbiol. 2018. PMID: 29114981
-
Structural and biochemical characterization of the exopolysaccharide deacetylase Agd3 required for Aspergillus fumigatus biofilm formation.Nat Commun. 2020 May 15;11(1):2450. doi: 10.1038/s41467-020-16144-5. Nat Commun. 2020. PMID: 32415073 Free PMC article.
-
Galactosaminogalactan (GAG) and its multiple roles in Aspergillus pathogenesis.Virulence. 2019 Dec;10(1):976-983. doi: 10.1080/21505594.2019.1568174. Epub 2019 Jan 22. Virulence. 2019. PMID: 30667338 Free PMC article. Review.
-
Aspergillus Biofilm In Vitro and In Vivo.Microbiol Spectr. 2015 Aug;3(4). doi: 10.1128/microbiolspec.MB-0017-2015. Microbiol Spectr. 2015. PMID: 26350307 Review.
Cited by
-
Unraveling the sugar code: the role of microbial extracellular glycans in plant-microbe interactions.J Exp Bot. 2021 Jan 20;72(1):15-35. doi: 10.1093/jxb/eraa414. J Exp Bot. 2021. PMID: 32929496 Free PMC article. Review.
-
Biofilm-Related, Time-Series Transcriptome and Genome Sequencing in Xylanase-Producing Aspergillus niger SJ1.ACS Omega. 2020 Jul 30;5(31):19737-19746. doi: 10.1021/acsomega.0c02501. eCollection 2020 Aug 11. ACS Omega. 2020. PMID: 32803069 Free PMC article.
-
Spt20, a Structural Subunit of the SAGA Complex, Regulates Aspergillus fumigatus Biofilm Formation, Asexual Development, and Virulence.Appl Environ Microbiol. 2022 Jan 11;88(1):e0153521. doi: 10.1128/AEM.01535-21. Epub 2021 Oct 20. Appl Environ Microbiol. 2022. PMID: 34669434 Free PMC article.
-
The Interface between Fungal Biofilms and Innate Immunity.Front Immunol. 2018 Jan 10;8:1968. doi: 10.3389/fimmu.2017.01968. eCollection 2017. Front Immunol. 2018. PMID: 29375581 Free PMC article. Review.
-
Biofilms 2018: A diversity of microbes and mechanisms.J Bacteriol. 2019 Feb 19;201(18):e00118-19. doi: 10.1128/JB.00118-19. Online ahead of print. J Bacteriol. 2019. PMID: 30782638 Free PMC article. Review.
References
-
- Abad A, Fernández-Molina JV, Bikandi J, Ramírez A, Margareto J, Sendino J, Hernando FL, Pontón J, Garaizar J, Rementeria A. 2010. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27:155–182. doi:10.1016/j.riam.2010.10.003. - DOI - PubMed
-
- Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, Xu W, Kravtsov I, Hoareau CMQ, Vanier G, Urb M, Campoli P, Al Abdallah Q, Lehoux M, Chabot JC, Ouimet M-C, Baptista SD, Fritz JH, Nierman WC, Latgé JP, Mitchell AP, Filler SG, Fontaine T, Sheppard DC. 2013. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog 9:e1003575. doi:10.1371/journal.ppat.1003575. - DOI - PMC - PubMed
-
- Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, van Kooyk Y, Bozza S, Moretti S, Schwarz F, Trichot C, Aebi M, Delepierre M, Elbim C, Romani L, Latgé JP. 2011. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog 7:e1002372. doi:10.1371/journal.ppat.1002372. - DOI - PMC - PubMed
-
- Takada H, Araki Y, Ito E. 1981. Structure of polygalactosamine produced by Aspergillus parasiticus. J Biochem 89:1265–1274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

