Inflammasomes as polyvalent cell death platforms
- PMID: 27048821
- PMCID: PMC11108487
- DOI: 10.1007/s00018-016-2204-3
Inflammasomes as polyvalent cell death platforms
Abstract
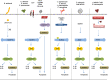
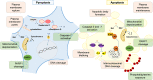
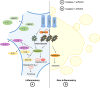
Inflammasomes are multi-protein platforms that are organized in the cytosol to cope with pathogens and cellular stress. The pattern recognition receptors NLRP1, NLRP3, NLRC4, AIM2 and Pyrin all assemble canonical platforms for caspase-1 activation, while caspase-11-dependent inflammasomes respond to intracellular Gram-negative pathogens. Inflammasomes are chiefly known for their roles in maturation and secretion of the inflammatory cytokines interleukin-(IL)1β and IL18, but they can also induce regulated cell death. Activation of caspases 1 and 11 in myeloid cells can trigger pyroptosis, a lytic and inflammatory cell death mode. Pyroptosis has been implicated in secretion of IL1β, IL18 and intracellular alarmins. Akin to these factors, it may have beneficial roles in controlling pathogen replication, but become detrimental in the context of chronic autoinflammatory diseases. Inflammasomes are increasingly implicated in induction of additional regulated cell death modes such as pyronecrosis and apoptosis. In this review, we overview recent advances in inflammasome-associated cell death research, illustrating the polyvalent roles of these macromolecular platforms in regulated cell death signaling.
Keywords: Apoptosis; Caspase-1; Caspase-11; Cell death; Inflammasome; Inflammation; Pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The intersection of cell death and inflammasome activation.Cell Mol Life Sci. 2016 Jun;73(11-12):2349-67. doi: 10.1007/s00018-016-2205-2. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066895 Free PMC article. Review.
-
Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis.Cold Spring Harb Perspect Biol. 2020 May 1;12(5):a036392. doi: 10.1101/cshperspect.a036392. Cold Spring Harb Perspect Biol. 2020. PMID: 31570336 Free PMC article. Review.
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
Mycobacterium tuberculosis infection of dendritic cells leads to partially caspase-1/11-independent IL-1β and IL-18 secretion but not to pyroptosis.PLoS One. 2012;7(7):e40722. doi: 10.1371/journal.pone.0040722. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911706 Free PMC article.
-
Pyroptotic cell death defends against intracellular pathogens.Immunol Rev. 2015 May;265(1):130-42. doi: 10.1111/imr.12287. Immunol Rev. 2015. PMID: 25879289 Free PMC article. Review.
Cited by
-
The role of regulated necrosis in endocrine diseases.Nat Rev Endocrinol. 2021 Aug;17(8):497-510. doi: 10.1038/s41574-021-00499-w. Epub 2021 Jun 16. Nat Rev Endocrinol. 2021. PMID: 34135504 Free PMC article. Review.
-
Inflammasome-more Injury in Cholestasis.Cell Mol Gastroenterol Hepatol. 2020;9(4):711-712. doi: 10.1016/j.jcmgh.2020.02.004. Epub 2020 Mar 7. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32156506 Free PMC article. No abstract available.
-
Off-Target Anti-Inflammatory Activity of the P2X7 Receptor Antagonist AZ11645373.Inflammation. 2017 Apr;40(2):530-536. doi: 10.1007/s10753-016-0499-8. Inflammation. 2017. PMID: 28101847 Free PMC article.
-
Mechanistic studies of the toxicity of zinc gluconate in the olfactory neuronal cell line Odora.Toxicol In Vitro. 2016 Sep;35:24-30. doi: 10.1016/j.tiv.2016.05.003. Epub 2016 May 12. Toxicol In Vitro. 2016. PMID: 27179668 Free PMC article.
-
Programmed Cell Death and Inflammation: Winter Is Coming.Trends Immunol. 2017 Oct;38(10):705-718. doi: 10.1016/j.it.2017.06.009. Epub 2017 Jul 19. Trends Immunol. 2017. PMID: 28734635 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

