Rapid, noninvasive quantitation of skin disease in systemic sclerosis using optical coherence elastography
- PMID: 27048877
- PMCID: PMC4837197
- DOI: 10.1117/1.JBO.21.4.046002
Rapid, noninvasive quantitation of skin disease in systemic sclerosis using optical coherence elastography
Abstract
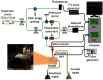
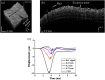
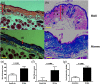
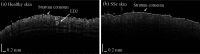
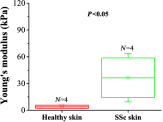
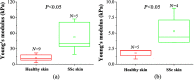
Systemic sclerosis (SSc) is a connective tissue disease that results in excessive accumulation of collagen in the skin and internal organs. Overall, SSc has a rare morbidity (276 cases per million adults in the United States), but has a 10-year survival rate of 55%. Currently, the modified Rodnan skin score (mRSS) is assessed by palpation on 17 sites on the body. However, the mRSS assessed score is subjective and may be influenced by the experience of the rheumatologists. In addition, the inherent elasticity of skin may bias the mRSS assessment in the early stage of SSc, such as oedematous. Optical coherence elastography (OCE) is a rapidly emerging technique, which can assess mechanical contrast in tissues with micrometer spatial resolution. In this work, the OCE technique is applied to assess the mechanical properties of skin in both control and bleomycin (BLM) induced SSc-like disease noninvasively. Young’s modulus of the BLM-SSc skin was found be significantly higher than that of normal skin, in both the in vivo and in vitro studies (p<0.05 p<0.05 ). Thus, OCE is able to differentiate healthy and fibrotic skin using mechanical contrast. It is a promising new technology for quantifying skin involvement in SSc in a rapid, unbiased, and noninvasive manner.
Figures

Similar articles
-
Translational optical coherence elastography for assessment of systemic sclerosis.J Biophotonics. 2019 Dec;12(12):e201900236. doi: 10.1002/jbio.201900236. Epub 2019 Aug 8. J Biophotonics. 2019. PMID: 31343837 Free PMC article.
-
Assessment of skin fibrosis in a murine model of systemic sclerosis with multifunctional optical coherence tomography.J Biomed Opt. 2025 Mar;30(3):036007. doi: 10.1117/1.JBO.30.3.036007. Epub 2025 Mar 27. J Biomed Opt. 2025. PMID: 40151216 Free PMC article.
-
How much of skin improvement over time in systemic sclerosis is due to normal ageing? A prospective study with shear-wave elastography.Arthritis Res Ther. 2020 Mar 18;22(1):50. doi: 10.1186/s13075-020-02150-x. Arthritis Res Ther. 2020. PMID: 32188488 Free PMC article.
-
Optical coherence elastography for tissue characterization: a review.J Biophotonics. 2015 Apr;8(4):279-302. doi: 10.1002/jbio.201400108. Epub 2014 Nov 20. J Biophotonics. 2015. PMID: 25412100 Free PMC article. Review.
-
High-resolution ultrasound imaging of skin involvement in systemic sclerosis: a systematic review.Rheumatol Int. 2021 Feb;41(2):285-295. doi: 10.1007/s00296-020-04761-8. Epub 2021 Jan 2. Rheumatol Int. 2021. PMID: 33386899
Cited by
-
Ultra-fast line-field low coherence holographic elastography using spatial phase shifting.Biomed Opt Express. 2017 Jan 23;8(2):993-1004. doi: 10.1364/BOE.8.000993. eCollection 2017 Feb 1. Biomed Opt Express. 2017. PMID: 28270998 Free PMC article.
-
Wave-based optical coherence elastography: The 10-year perspective.Prog Biomed Eng (Bristol). 2022 Jan;4(1):012007. doi: 10.1088/2516-1091/ac4512. Epub 2022 Jan 14. Prog Biomed Eng (Bristol). 2022. PMID: 35187403 Free PMC article.
-
Deep Learning Classification of Systemic Sclerosis Skin Using the MobileNetV2 Model.IEEE Open J Eng Med Biol. 2021 Mar 17;2:104-110. doi: 10.1109/OJEMB.2021.3066097. eCollection 2021. IEEE Open J Eng Med Biol. 2021. PMID: 35402975 Free PMC article.
-
Translational optical coherence elastography for assessment of systemic sclerosis.J Biophotonics. 2019 Dec;12(12):e201900236. doi: 10.1002/jbio.201900236. Epub 2019 Aug 8. J Biophotonics. 2019. PMID: 31343837 Free PMC article.
-
1.7-Micron Optical Coherence Tomography Angiography for Characterization of Skin Lesions-A Feasibility Study.IEEE Trans Med Imaging. 2021 Sep;40(9):2507-2512. doi: 10.1109/TMI.2021.3081066. Epub 2021 Aug 31. IEEE Trans Med Imaging. 2021. PMID: 33999817 Free PMC article.
References
-
- Denton C. P., “Systemic sclerosis: from pathogenesis to targeted therapy,” Clin. Exp. Rheumatol. 33(4 Suppl 92), S3–S7 (2015).CERHDP - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

