Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL
- PMID: 27048913
- PMCID: PMC4823827
- DOI: 10.1038/ncomms11190
Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL
Abstract
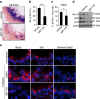
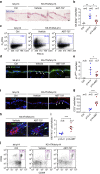
Senescent cells, formed in response to physiological and oncogenic stresses, facilitate protection from tumourigenesis and aid in tissue repair. However, accumulation of such cells in tissues contributes to age-related pathologies. Resistance of senescent cells to apoptotic stimuli may contribute to their accumulation, yet the molecular mechanisms allowing their prolonged viability are poorly characterized. Here we show that senescent cells upregulate the anti-apoptotic proteins BCL-W and BCL-XL. Joint inhibition of BCL-W and BCL-XL by siRNAs or the small-molecule ABT-737 specifically induces apoptosis in senescent cells. Notably, treatment of mice with ABT-737 efficiently eliminates senescent cells induced by DNA damage in the lungs as well as senescent cells formed in the epidermis by activation of p53 through transgenic p14(ARF). Elimination of senescent cells from the epidermis leads to an increase in hair-follicle stem cell proliferation. The finding that senescent cells can be eliminated pharmacologically paves the way to new strategies for the treatment of age-related pathologies.
Figures



References
-
- Munoz-Espin D. & Serrano M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

