Stimulation of ipt overexpression as a tool to elucidate the role of cytokinins in high temperature responses of Arabidopsis thaliana
- PMID: 27049021
- PMCID: PMC4861028
- DOI: 10.1093/jxb/erw129
Stimulation of ipt overexpression as a tool to elucidate the role of cytokinins in high temperature responses of Arabidopsis thaliana
Abstract
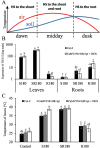
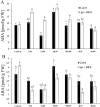
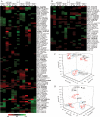
Cytokinins (CKs) are phytohormones regulating plant growth and development as well as response to the environment. In order to evaluate their function in heat stress (HS) responses, the effect of CK elevation was determined during three types of HS - targeted to shoots, targeted to roots and applied to the whole plant. The early (30min) and longer term (3h) responses were followed at the hormonal, transcriptomic and proteomic levels in Arabidopsis transformants with dexamethasone-inducible expression of the CK biosynthetic gene isopentenyltransferase (ipt) and the corresponding wild-type (Col-0). Combination of hormonal and phenotypic analyses showed transient up-regulation of the CK/abscisic acid ratio, which controls stomatal aperture, to be more pronounced in the transformant. HS responses of the root proteome and Rubisco-immunodepleted leaf proteome were followed using 2-D gel electrophoresis and MALDI-TOF/TOF. More than 100 HS-responsive proteins were detected, most of them being modulated by CK increase. Proteome and transcriptome analyses demonstrated that CKs have longer term positive effects on the stress-related proteins and transcripts, as well as on the photosynthesis-related ones. Transient accumulation of CKs and stimulation of their signal transduction in tissue(s) not exposed to HS indicate that they are involved in plant stress responses.
Keywords: Abscisic acid; Arabidopsis thaliana; cytokinin; heat stress; isopentenyltransferase; proteome..
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




Similar articles
-
Proteome and metabolome profiling of cytokinin action in Arabidopsis identifying both distinct and similar responses to cytokinin down- and up-regulation.J Exp Bot. 2013 Nov;64(14):4193-206. doi: 10.1093/jxb/ert227. Epub 2013 Sep 24. J Exp Bot. 2013. PMID: 24064926 Free PMC article.
-
Proteome analysis in Arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis.Plant Physiol. 2013 Feb;161(2):918-30. doi: 10.1104/pp.112.202853. Epub 2012 Dec 3. Plant Physiol. 2013. PMID: 23209126 Free PMC article.
-
Effect on shoot water relations, and cytokinin and abscisic acid levels of inducing expression of a gene coding for isopentenyltransferase in roots of transgenic tobacco plants.J Exp Bot. 2010 Aug;61(13):3709-17. doi: 10.1093/jxb/erq182. Epub 2010 Jul 19. J Exp Bot. 2010. PMID: 20643808
-
Molecular basis for cytokinin biosynthesis.Phytochemistry. 2009 Mar;70(4):444-9. doi: 10.1016/j.phytochem.2009.02.007. Epub 2009 Mar 11. Phytochemistry. 2009. PMID: 19285326 Review.
-
Phytohormones and their crosstalk in regulating stomatal development and patterning.J Exp Bot. 2021 Mar 29;72(7):2356-2370. doi: 10.1093/jxb/erab034. J Exp Bot. 2021. PMID: 33512461 Review.
Cited by
-
Cytokinin and abiotic stress tolerance -What has been accomplished and the way forward?Front Genet. 2022 Aug 9;13:943025. doi: 10.3389/fgene.2022.943025. eCollection 2022. Front Genet. 2022. Retraction in: Front Genet. 2024 Mar 19;15:1399259. doi: 10.3389/fgene.2024.1399259. PMID: 36017502 Free PMC article. Retracted. Review.
-
Roles of phytohormone changes in the grain yield of rice plants exposed to heat: a review.PeerJ. 2019 Nov 19;7:e7792. doi: 10.7717/peerj.7792. eCollection 2019. PeerJ. 2019. PMID: 31763066 Free PMC article.
-
Light Regulates the Cytokinin-Dependent Cold Stress Responses in Arabidopsis.Front Plant Sci. 2021 Feb 4;11:608711. doi: 10.3389/fpls.2020.608711. eCollection 2020. Front Plant Sci. 2021. PMID: 33613584 Free PMC article.
-
Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change.Front Plant Sci. 2021 Feb 11;11:627969. doi: 10.3389/fpls.2020.627969. eCollection 2020. Front Plant Sci. 2021. PMID: 33643337 Free PMC article. Review.
-
Research Progress on the Roles of Cytokinin in Plant Response to Stress.Int J Mol Sci. 2020 Sep 8;21(18):6574. doi: 10.3390/ijms21186574. Int J Mol Sci. 2020. PMID: 32911801 Free PMC article. Review.
References
-
- Ache P, Bauer H, Kollist H, Al-Rasheid KAS, Lautner S, Hartung W, Hedrich R. 2010. Stomatal action directly feeds back on leaf turgor: New insights into the regulation of the plant water status from non-invasive pressure probe measurements. The Plant Journal 62, 1072–1082. - PubMed
-
- Baldrianová J, Černý M, Novák J, Jedelský PL, Divíšková E, Brzobohatý B. 2015. Arabidopsis proteome responses to the smoke-derived growth regulator karrikin. Journal of Proteomics 120, 7–20. - PubMed
-
- Bevan M, Bancroft I, Bent E, et al. 1998. Analysis of 1.9Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana . Nature 391, 485–488. - PubMed
-
- Caers M, Rudelsheim P, Van Onckelen H. 1985. Effect of heat stress on photosynthetic activity and chloroplast infrastructure in correlation with endogenous cytokinin concentration in maize seedlings. Plant & Cell Physiology 26, 47–52.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

