Simultaneous blockade of VEGF and Dll4 by HD105, a bispecific antibody, inhibits tumor progression and angiogenesis
- PMID: 27049350
- PMCID: PMC4968104
- DOI: 10.1080/19420862.2016.1171432
Simultaneous blockade of VEGF and Dll4 by HD105, a bispecific antibody, inhibits tumor progression and angiogenesis
Abstract
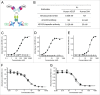
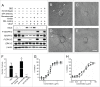
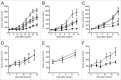
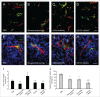
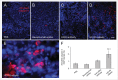
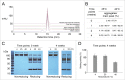
Several angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) signaling pathway have been approved for cancer treatment. However, VEGF inhibitors alone were shown to promote tumor invasion and metastasis by increasing intratumoral hypoxia in some preclinical and clinical studies. Emerging reports suggest that Delta-like ligand 4 (Dll4) is a promising target of angiogenesis inhibition to augment the effects of VEGF inhibitors. To evaluate the effects of simultaneous blockade against VEGF and Dll4, we developed a bispecific antibody, HD105, targeting VEGF and Dll4. The HD105 bispecific antibody, which is composed of an anti-VEGF antibody (bevacizumab-similar) backbone C-terminally linked with a Dll4-targeting single-chain variable fragment, showed potent binding affinities against VEGF (KD: 1.3 nM) and Dll4 (KD: 30 nM). In addition, the HD105 bispecific antibody competitively inhibited the binding of ligands to their receptors, i.e., VEGF to VEGFR2 (EC50: 2.84 ± 0.41 nM) and Dll4 to Notch1 (EC50: 1.14 ± 0.06 nM). Using in vitro cell-based assays, we found that HD105 effectively blocked both the VEGF/VEGFR2 and Dll4/Notch1 signaling pathways in endothelial cells, resulting in a conspicuous inhibition of endothelial cell proliferation and sprouting. HD105 also suppressed Dll4-induced Notch1-dependent activation of the luciferase gene. In vivo xenograft studies demonstrated that HD105 more efficiently inhibited the tumor progression of human A549 lung and SCH gastric cancers than an anti-VEGF antibody or anti-Dll4 antibody alone. In conclusion, HD105 may be a novel therapeutic bispecific antibody for cancer treatment.
Keywords: Anti-angiogenesis; VEGF; anti-cancer; biologics; delta-like ligand; therapeutic antibody.
Figures
Similar articles
-
ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models.Int J Mol Sci. 2020 Dec 29;22(1):241. doi: 10.3390/ijms22010241. Int J Mol Sci. 2020. PMID: 33383646 Free PMC article.
-
Dll4 Blockade in Stromal Cells Mediates Antitumor Effects in Preclinical Models of Ovarian Cancer.Cancer Res. 2015 Oct 1;75(19):4086-96. doi: 10.1158/0008-5472.CAN-14-3773. Epub 2015 Sep 16. Cancer Res. 2015. PMID: 26377940
-
The bispecific antibody HB-32, blockade of both VEGF and DLL4 shows potent anti-angiogenic activity in vitro and anti-tumor activity in breast cancer xenograft models.Exp Cell Res. 2019 Jul 15;380(2):141-148. doi: 10.1016/j.yexcr.2019.04.025. Epub 2019 Apr 26. Exp Cell Res. 2019. PMID: 31034805
-
Delta-like 4/Notch signaling and its therapeutic implications.Clin Cancer Res. 2007 Dec 15;13(24):7243-6. doi: 10.1158/1078-0432.CCR-07-1393. Clin Cancer Res. 2007. PMID: 18094402 Review.
-
The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth.Nat Rev Cancer. 2007 May;7(5):327-31. doi: 10.1038/nrc2130. Nat Rev Cancer. 2007. PMID: 17457300 Review.
Cited by
-
Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting?Front Oncol. 2018 Jul 24;8:267. doi: 10.3389/fonc.2018.00267. eCollection 2018. Front Oncol. 2018. PMID: 30087852 Free PMC article. Review.
-
Immunotherapeutic progress and application of bispecific antibody in cancer.Front Immunol. 2022 Oct 20;13:1020003. doi: 10.3389/fimmu.2022.1020003. eCollection 2022. Front Immunol. 2022. PMID: 36341333 Free PMC article. Review.
-
Synergistic antitumor activity of a DLL4/VEGF bispecific therapeutic antibody in combination with irinotecan in gastric cancer.BMB Rep. 2020 Nov;53(10):533-538. doi: 10.5483/BMBRep.2020.53.10.103. BMB Rep. 2020. PMID: 32580836 Free PMC article.
-
A Vascular Endothelial Growth Factor-Dependent Sprouting Angiogenesis Assay Based on an In Vitro Human Blood Vessel Model for the Study of Anti-Angiogenic Drugs.EBioMedicine. 2018 Jan;27:225-236. doi: 10.1016/j.ebiom.2017.12.014. Epub 2017 Dec 20. EBioMedicine. 2018. PMID: 29289530 Free PMC article.
-
Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies.BioDrugs. 2018 Oct;32(5):441-464. doi: 10.1007/s40259-018-0299-9. BioDrugs. 2018. PMID: 30132211 Free PMC article. Review.
References
-
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999; 13:9-22; PMID:9872925 - PubMed
-
- Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med 2012; 2:a006577; PMID:23028128; http://dx.doi.org/10.1101/cshperspect.a006577 - DOI - PMC - PubMed
-
- Casak SJ, Fashoyin-Aje I, Lemery SJ, Zhang L, Jin R, Li H, Zhao L, Zhao H, Zhang H, Chen H, et al.. FDA Approval Summary: Ramucirumab for Gastric Cancer. Clin Cancer Res 2015; 21:3372-6; PMID:26048277; http://dx.doi.org/10.1158/1078-0432.CCR-15-0600 - DOI - PubMed
-
- Trichonas G, Kaiser PK. Aflibercept for the treatment of age-related macular degeneration. Ophthalmol Ther. 2013; 2:89-98; PMID:25135809; http://dx.doi.org/10.1007/s40123-013-0015-2 - DOI - PMC - PubMed
-
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8:592-603; PMID:18650835; http://dx.doi.org/10.1038/nrc2442 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
