Synthesis of Dense and Chiral Dendritic Polyols Using Glyconanosynthon Scaffolds
- PMID: 27049377
- PMCID: PMC6274151
- DOI: 10.3390/molecules21040448
Synthesis of Dense and Chiral Dendritic Polyols Using Glyconanosynthon Scaffolds
Abstract
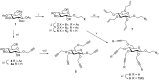
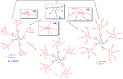
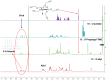
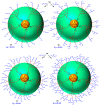
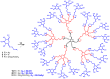
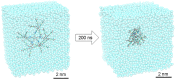
Most classical dendrimers are frequently built-up from identical repeating units of low valency (usually AB2 monomers). This strategy necessitates several generations to achieve a large number of surface functionalities. In addition, these typical monomers are achiral. We propose herein the use of sugar derivatives consisting of several and varied functionalities with their own individual intrinsic chirality as both scaffolds/core as well as repeating units. This approach allows the construction of chiral, dense dendrimers with a large number of surface groups at low dendrimer generations. Perpropargylated β-D-glucopyranoside, serving as an A5 core, together with various derivatives, such as 2-azidoethyl tetra-O-allyl-β-D-glucopyranoside, serving as an AB4 repeating moiety, were utilized to construct chiral dendrimers using "click chemistry" (CuAAC reaction). These were further modified by thiol-ene and thiol-yne click reactions with alcohols to provide dendritic polyols. Molecular dynamic simulation supported the assumption that the resulting polyols have a dense structure.
Keywords: CuAAAC; carbohydrate; click chemistry; dendrimer; glycodendrimer; thiol-ene; thiol-yne.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

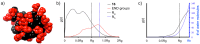
Similar articles
-
Facile and Efficient Synthesis of Carbosiloxane Dendrimers via Orthogonal Click Chemistry Between Thiol and Ene.Macromol Rapid Commun. 2016 Feb;37(4):318-22. doi: 10.1002/marc.201500607. Epub 2015 Dec 16. Macromol Rapid Commun. 2016. PMID: 26676283
-
Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene "click" chemistry.J Am Chem Soc. 2008 Apr 16;130(15):5062-4. doi: 10.1021/ja8006325. Epub 2008 Mar 20. J Am Chem Soc. 2008. PMID: 18355008
-
Thiol-ene click chemistry.Angew Chem Int Ed Engl. 2010 Feb 22;49(9):1540-73. doi: 10.1002/anie.200903924. Angew Chem Int Ed Engl. 2010. PMID: 20166107 Review.
-
Dendron synthesis and carbohydrate immobilization on a biomaterial surface by a double-click reaction.Org Lett. 2014 Mar 7;16(5):1298-301. doi: 10.1021/ol403476z. Epub 2014 Feb 19. Org Lett. 2014. PMID: 24552198
-
Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials.Acc Chem Res. 2012 Apr 17;45(4):630-40. doi: 10.1021/ar200235m. Epub 2011 Dec 8. Acc Chem Res. 2012. PMID: 22148925 Review.
Cited by
-
Discovery of highly potent and selective 7-ethyl-10-hydroxycamptothecin-glucose conjugates as potential anti-colorectal cancer agents.Front Pharmacol. 2022 Nov 23;13:1014854. doi: 10.3389/fphar.2022.1014854. eCollection 2022. Front Pharmacol. 2022. PMID: 36506586 Free PMC article.
-
Innovative Strategy for Truly Reversible Capture of Polluting Gases-Application to Carbon Dioxide.Int J Mol Sci. 2023 Nov 17;24(22):16463. doi: 10.3390/ijms242216463. Int J Mol Sci. 2023. PMID: 38003653 Free PMC article. Review.
-
Special Issue: "Functional Dendrimers".Molecules. 2016 Aug 9;21(8):1035. doi: 10.3390/molecules21081035. Molecules. 2016. PMID: 27517890 Free PMC article.
References
-
- Tomalia D.A., Naylor A.M., Goddard W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. 1990;29:138–175. doi: 10.1002/anie.199001381. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

