The Effect of Early Goal-Directed Therapy on Outcome in Adult Severe Sepsis and Septic Shock Patients: A Meta-Analysis of Randomized Clinical Trials
- PMID: 27049857
- PMCID: PMC4956677
- DOI: 10.1213/ANE.0000000000001278
The Effect of Early Goal-Directed Therapy on Outcome in Adult Severe Sepsis and Septic Shock Patients: A Meta-Analysis of Randomized Clinical Trials
Abstract
Background: Whether early goal-directed therapy (EGDT) improves outcome in severe sepsis and septic shock remains unclear. We performed a meta-analysis of existing clinical trials to examine whether EGDT improved outcome in the resuscitation of adult sepsis patients compared with control care.
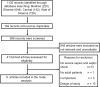
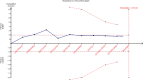
Methods: We searched for eligible studies using MEDLINE, Elsevier, Cochrane Central Register of Controlled Trials, and Web of Science databases. Studies were eligible if they compared the effects of EGDT versus control care on mortality in adult patients with severe sepsis and septic shock. Two reviewers extracted data independently. Data including mortality, sample size of the patients with severe sepsis and septic shock, and resuscitation end points were extracted. Data were analyzed using methods recommended by the Cochrane Collaboration Review Manager 4.2 software. Random errors were evaluated by trial sequential analysis (TSA).
Results: Nine studies compared EGDT with control care, and 5202 severe sepsis and septic shock patients were included. A nonsignificant trend toward reduction in the longest all-cause mortality was observed in the EGDT group compared with control care (relative risk, 0.89; 99% confidence interval, 0.74-1.07; P = 0.10). However, EGDT significantly reduced intensive care unit mortality in severe sepsis and septic shock patients (relative risk, 0.72; 99% confidence interval, 0.57-0.90; P = 0.0002). TSA indicated lack of firm evidence for a beneficial effect.
Conclusions: In this meta-analysis, a nonsignificant trend toward reduction in the longest all-cause mortality in patients resuscitated with EGDT was noted. However, EGDT significantly reduced intensive care unit mortality in severe sepsis and septic shock patients. TSA indicated a lack of firm evidence for the results. More powered, randomized controlled trials are needed to determine the effects.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Early Goal-Directed Therapy in Severe Sepsis and Septic Shock: A Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials.J Intensive Care Med. 2018 May;33(5):296-309. doi: 10.1177/0885066616671710. Epub 2016 Oct 22. J Intensive Care Med. 2018. PMID: 27756870
-
Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials.Crit Care. 2014 Dec 15;18(6):702. doi: 10.1186/s13054-014-0702-y. Crit Care. 2014. PMID: 25499187 Free PMC article.
-
The effect of early goal-directed therapy on mortality in patients with severe sepsis and septic shock: a meta-analysis.J Surg Res. 2016 May 15;202(2):389-97. doi: 10.1016/j.jss.2015.12.048. Epub 2016 Jan 4. J Surg Res. 2016. PMID: 27229114
-
Early goal-directed therapy in the management of severe sepsis or septic shock in adults: a meta-analysis of randomized controlled trials.BMC Med. 2015 Apr 3;13:71. doi: 10.1186/s12916-015-0312-9. BMC Med. 2015. PMID: 25885654 Free PMC article.
-
Early goal-directed therapy reduces mortality in adult patients with severe sepsis and septic shock: Systematic review and meta-analysis.Indian J Crit Care Med. 2015 Jul;19(7):401-11. doi: 10.4103/0972-5229.160281. Indian J Crit Care Med. 2015. PMID: 26180433 Free PMC article. Review.
Cited by
-
Prognostic nutrition index is associated with the all-cause mortality in sepsis patients: A retrospective cohort study.J Clin Lab Anal. 2022 Apr;36(4):e24297. doi: 10.1002/jcla.24297. Epub 2022 Feb 20. J Clin Lab Anal. 2022. PMID: 35187716 Free PMC article.
-
Health economic evaluations of sepsis interventions in critically ill adult patients: a systematic review.J Intensive Care. 2020 Jan 8;8:5. doi: 10.1186/s40560-019-0412-2. eCollection 2020. J Intensive Care. 2020. PMID: 31934338 Free PMC article.
-
The impact of selenium administration on severe sepsis or septic shock: a meta-analysis of randomized controlled trials.Afr Health Sci. 2021 Mar;21(1):277-285. doi: 10.4314/ahs.v21i1.36. Afr Health Sci. 2021. PMID: 34394308 Free PMC article.
-
Critical Care Medicine 2017: Bigger Picture, Better Future.Chin Med J (Engl). 2017 May 20;130(10):1135-1136. doi: 10.4103/0366-6999.205866. Chin Med J (Engl). 2017. PMID: 28485310 Free PMC article. No abstract available.
-
Early outcome of early-goal directed therapy for patients with sepsis or septic shock: a systematic review and meta-analysis of randomized controlled trials.Oncotarget. 2017 Apr 18;8(16):27510-27519. doi: 10.18632/oncotarget.15550. Oncotarget. 2017. PMID: 28460438 Free PMC article.
References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. - PubMed
-
- Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–16. - PubMed
-
- Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, Osborn T, Lemeshow S, Chiche JD, Artigas A, Dellinger RP. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014;40:1623–33. - PubMed
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

