Reductive carboxylation supports redox homeostasis during anchorage-independent growth
- PMID: 27049945
- PMCID: PMC4860952
- DOI: 10.1038/nature17393
Reductive carboxylation supports redox homeostasis during anchorage-independent growth
Abstract
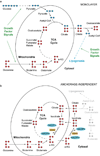
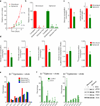
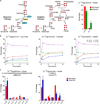
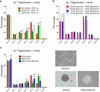
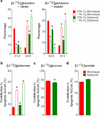
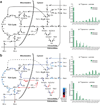
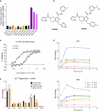
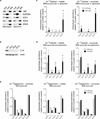
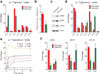
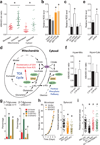
Cells receive growth and survival stimuli through their attachment to an extracellular matrix (ECM). Overcoming the addiction to ECM-induced signals is required for anchorage-independent growth, a property of most malignant cells. Detachment from ECM is associated with enhanced production of reactive oxygen species (ROS) owing to altered glucose metabolism. Here we identify an unconventional pathway that supports redox homeostasis and growth during adaptation to anchorage independence. We observed that detachment from monolayer culture and growth as anchorage-independent tumour spheroids was accompanied by changes in both glucose and glutamine metabolism. Specifically, oxidation of both nutrients was suppressed in spheroids, whereas reductive formation of citrate from glutamine was enhanced. Reductive glutamine metabolism was highly dependent on cytosolic isocitrate dehydrogenase-1 (IDH1), because the activity was suppressed in cells homozygous null for IDH1 or treated with an IDH1 inhibitor. This activity occurred in absence of hypoxia, a well-known inducer of reductive metabolism. Rather, IDH1 mitigated mitochondrial ROS in spheroids, and suppressing IDH1 reduced spheroid growth through a mechanism requiring mitochondrial ROS. Isotope tracing revealed that in spheroids, isocitrate/citrate produced reductively in the cytosol could enter the mitochondria and participate in oxidative metabolism, including oxidation by IDH2. This generates NADPH in the mitochondria, enabling cells to mitigate mitochondrial ROS and maximize growth. Neither IDH1 nor IDH2 was necessary for monolayer growth, but deleting either one enhanced mitochondrial ROS and reduced spheroid size, as did deletion of the mitochondrial citrate transporter protein. Together, the data indicate that adaptation to anchorage independence requires a fundamental change in citrate metabolism, initiated by IDH1-dependent reductive carboxylation and culminating in suppression of mitochondrial ROS.
Figures



Comment in
-
Coping with the metabolic stress of leaving home.Cell Res. 2016 Jul;26(7):757-8. doi: 10.1038/cr.2016.61. Epub 2016 May 24. Cell Res. 2016. PMID: 27216116 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

