N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells
- PMID: 27050099
- PMCID: PMC4829466
- DOI: 10.1016/j.ccell.2016.03.001
N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells
Abstract
MYCN amplification and overexpression are common in neuroendocrine prostate cancer (NEPC). However, the impact of aberrant N-Myc expression in prostate tumorigenesis and the cellular origin of NEPC have not been established. We define N-Myc and activated AKT1 as oncogenic components sufficient to transform human prostate epithelial cells to prostate adenocarcinoma and NEPC with phenotypic and molecular features of aggressive, late-stage human disease. We directly show that prostate adenocarcinoma and NEPC can arise from a common epithelial clone. Further, N-Myc is required for tumor maintenance, and destabilization of N-Myc through Aurora A kinase inhibition reduces tumor burden. Our findings establish N-Myc as a driver of NEPC and a target for therapeutic intervention.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
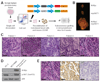




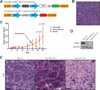
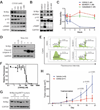
Comment in
-
Defining and Targeting the Oncogenic Drivers of Neuroendocrine Prostate Cancer.Cancer Cell. 2016 Apr 11;29(4):431-432. doi: 10.1016/j.ccell.2016.03.023. Cancer Cell. 2016. PMID: 27070695 Free PMC article.
-
Re: N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells.J Urol. 2016 Nov;196(5):1584-1585. doi: 10.1016/j.juro.2016.08.023. Epub 2016 Aug 23. J Urol. 2016. PMID: 27751495 No abstract available.
References
-
- Beltran H. The N-myc Oncogene: Maximizing its Targets, Regulation, and Therapeutic Potential. Molecular cancer research : MCR. 2014;12:815–822. - PubMed
-
- Boutros PC, Fraser M, Harding NJ, de Borja R, Trudel D, Lalonde E, Meng A, Hennings-Yeomans PH, McPherson A, Sabelnykova VY, et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nature genetics. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA181242/CA/NCI NIH HHS/United States
- U54 HG006097/HG/NHGRI NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- K99 CA184397/CA/NCI NIH HHS/United States
- T32 CA009056/CA/NCI NIH HHS/United States
- R01 CA180778/CA/NCI NIH HHS/United States
- R03 CA230819/CA/NCI NIH HHS/United States
- R01 CA195505/CA/NCI NIH HHS/United States
- K08 NS079485/NS/NINDS NIH HHS/United States
- R01 GM109031/GM/NIGMS NIH HHS/United States
- U01 CA164188/CA/NCI NIH HHS/United States
- P50 CA092131/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- T32 CA009120/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R00 CA184397/CA/NCI NIH HHS/United States
- R01 CA172603/CA/NCI NIH HHS/United States
- U54 HL127365/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

