Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells
- PMID: 27050147
- PMCID: PMC5078009
- DOI: 10.18632/oncotarget.8515
Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells
Abstract
Matrix stiffness as an important physical attribute of extracellular matrix exerts significant impacts on biological behaviors of cancer cells such as growth, proliferation, motility, metabolism and invasion. However, its influence on cancer stemness still remains elusive. Here, we explore whether matrix stiffness-mediated effects on stemness characteristics occur in HCC cells. As the substrate stiffness increased, HCC cells exhibited high proportion of cells with CD133(+)/EpCAM(+), high expression levels of CD133, EpCAM, Nanog and SOX2, greater self-renewing ability and oxaliplatin resistance. Simultaneously, their phosphorylation levels of Akt and mTOR, as well as p-4E-BP and SOX2 expressions were also obviously upregulated. Conversely, knockdown of integrin β1 partially attenuated higher stiffness-mediated stemness characteristics in HCC cells, and reversed the phosphorylation levels of Akt and mTOR, and expressions of p-4E-BP and SOX2, suggesting that integrin β1 may deliver higher stiffness signal into HCC cells and activate mTOR signaling pathway. Additionally, mTOR inhibitor suppressed the mTOR phosphorylation level and expression levels of p-4E-BP and SOX2 in HCC cells grown on higher stiffness substrate, as well as depressed their stemness properties significantly, favoring a regulating role of mTOR signaling pathway in matrix stiffness-mediated effects on stemness. In summary, matrix stiffness may be involved in the process of stemness regulation via activating integrin β1/Akt/mTOR/SOX2 signaling pathway. To the best of our knowledge, this study first reveals a novel regulating pathway to direct the stemness characteristics in HCC cells.
Keywords: hepatocellular carcinoma; integrin β1; mTOR; matrix stiffness; stemness.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

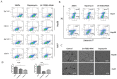

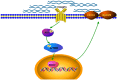
Similar articles
-
Cyclin G1 expands liver tumor-initiating cells by Sox2 induction via Akt/mTOR signaling.Mol Cancer Ther. 2013 Sep;12(9):1796-804. doi: 10.1158/1535-7163.MCT-13-0099. Epub 2013 Jun 26. Mol Cancer Ther. 2013. PMID: 23804702
-
Elevated PDK1 Expression Drives PI3K/AKT/MTOR Signaling Promotes Radiation-Resistant and Dedifferentiated Phenotype of Hepatocellular Carcinoma.Cells. 2020 Mar 18;9(3):746. doi: 10.3390/cells9030746. Cells. 2020. PMID: 32197467 Free PMC article.
-
Docetaxel promotes cell apoptosis and decreases SOX2 expression in CD133‑expressing hepatocellular carcinoma stem cells by suppressing the PI3K/AKT signaling pathway.Oncol Rep. 2019 Feb;41(2):1067-1074. doi: 10.3892/or.2018.6891. Epub 2018 Nov 27. Oncol Rep. 2019. PMID: 30483804
-
[Research advances in the mammalian target of rapamycin signaling pathway and its inhibitors in treatment of hepatocellular carcinoma].Zhonghua Gan Zang Bing Za Zhi. 2018 Jan 20;26(1):77-80. doi: 10.3760/cma.j.issn.1007-3418.2018.01.018. Zhonghua Gan Zang Bing Za Zhi. 2018. PMID: 29804369 Review. Chinese.
-
Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies.Cells. 2020 May 26;9(6):1331. doi: 10.3390/cells9061331. Cells. 2020. PMID: 32466488 Free PMC article. Review.
Cited by
-
Tim-1-mediated extracellular matrix promotes the development of hepatocellular carcinoma.Acta Biochim Biophys Sin (Shanghai). 2024 Oct 23;56(12):1761-1773. doi: 10.3724/abbs.2024191. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 39444345 Free PMC article.
-
The first embryo, the origin of cancer and animal phylogeny. V. Cancer stem cells as the unifying biomechanical principle between embryology and oncology.Mechanobiol Med. 2024 Dec 5;3(1):100110. doi: 10.1016/j.mbm.2024.100110. eCollection 2025 Mar. Mechanobiol Med. 2024. PMID: 40396136 Free PMC article. Review.
-
Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications.Nat Rev Gastroenterol Hepatol. 2022 Jan;19(1):26-44. doi: 10.1038/s41575-021-00508-3. Epub 2021 Sep 9. Nat Rev Gastroenterol Hepatol. 2022. PMID: 34504325 Review.
-
Effects of biomechanical forces on the biological behavior of cancer stem cells.J Cancer. 2021 Aug 8;12(19):5895-5902. doi: 10.7150/jca.60893. eCollection 2021. J Cancer. 2021. PMID: 34476003 Free PMC article. Review.
-
Chemotherapy-Enriched THBS2-Deficient Cancer Stem Cells Drive Hepatocarcinogenesis through Matrix Softness Induced Histone H3 Modifications.Adv Sci (Weinh). 2021 Jan 4;8(5):2002483. doi: 10.1002/advs.202002483. eCollection 2021 Mar. Adv Sci (Weinh). 2021. PMID: 33717837 Free PMC article.
References
-
- Gao J, Xie L, Yang WS, Zhang W, Gao S, Wang J, Xiang YB. Risk factors of hepatocellular carcinoma--current status and perspectives. Asian Pac J Cancer Prev. 2012;13:743–752. - PubMed
-
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonal-coholic fatty liver disease. Hepatology. 2009;51:454–462. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

