Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia
- PMID: 27050191
- PMCID: PMC5405769
- DOI: 10.1016/j.jacc.2016.03.520
Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia
Abstract
Background: Approximately 7% of American adults have severe hypercholesterolemia (untreated low-density lipoprotein [LDL] cholesterol ≥190 mg/dl), which may be due to familial hypercholesterolemia (FH). Lifelong LDL cholesterol elevations in FH mutation carriers may confer coronary artery disease (CAD) risk beyond that captured by a single LDL cholesterol measurement.
Objectives: This study assessed the prevalence of an FH mutation among those with severe hypercholesterolemia and determined whether CAD risk varies according to mutation status beyond the observed LDL cholesterol level.
Methods: Three genes causative for FH (LDLR, APOB, and PCSK9) were sequenced in 26,025 participants from 7 case-control studies (5,540 CAD case subjects, 8,577 CAD-free control subjects) and 5 prospective cohort studies (11,908 participants). FH mutations included loss-of-function variants in LDLR, missense mutations in LDLR predicted to be damaging, and variants linked to FH in ClinVar, a clinical genetics database.
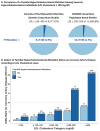
Results: Among 20,485 CAD-free control and prospective cohort participants, 1,386 (6.7%) had LDL cholesterol ≥190 mg/dl; of these, only 24 (1.7%) carried an FH mutation. Within any stratum of observed LDL cholesterol, risk of CAD was higher among FH mutation carriers than noncarriers. Compared with a reference group with LDL cholesterol <130 mg/dl and no mutation, participants with LDL cholesterol ≥190 mg/dl and no FH mutation had a 6-fold higher risk for CAD (odds ratio: 6.0; 95% confidence interval: 5.2 to 6.9), whereas those with both LDL cholesterol ≥190 mg/dl and an FH mutation demonstrated a 22-fold increased risk (odds ratio: 22.3; 95% confidence interval: 10.7 to 53.2). In an analysis of participants with serial lipid measurements over many years, FH mutation carriers had higher cumulative exposure to LDL cholesterol than noncarriers.
Conclusions: Among participants with LDL cholesterol ≥190 mg/dl, gene sequencing identified an FH mutation in <2%. However, for any observed LDL cholesterol, FH mutation carriers had substantially increased risk for CAD.
Keywords: coronary artery disease; gene sequencing; genetics; low-density lipoprotein cholesterol.
Copyright © 2016 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr Khera is supported by an ACC/Merck Fellowship award and reported consulting fees from Merck and Amarin. Dr Peloso is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL125751. Dr Kessler is supported by a DZHK Rotation Grant. Dr Rader reported consulting fees from Aegerion, Alnylam, Eli Lilly, Pfizer, and Novartis, is an inventor on a patent related to lomitapide that is owned by the University of Pennsylvania and licensed to Aegerion, and is a co-founder of VascularStrategies and Staten Biotechnology. Dr Kathiresan has received grants from Bayer Healthcare, Aegerion, and Regeneron and reported consulting fees from Merck, Quest Diagnostics, Genomics PLC, and Eli Lilly.
Figures



Comment in
-
Dyslipidaemia: FH genes, beyond LDL-C, predict CAD.Nat Rev Cardiol. 2016 Jun;13(6):314. doi: 10.1038/nrcardio.2016.62. Epub 2016 Apr 21. Nat Rev Cardiol. 2016. PMID: 27098141 No abstract available.
-
Familial Hypercholesterolemia: Now Part of Cardiovascular Disease Genetic Epidemiology Research.J Am Coll Cardiol. 2016 Jun 7;67(22):2590-2. doi: 10.1016/j.jacc.2016.03.567. J Am Coll Cardiol. 2016. PMID: 27256830 No abstract available.
-
The Independent Malignity of Familial Hypercholesterolemia.J Am Coll Cardiol. 2017 Feb 14;69(6):753-754. doi: 10.1016/j.jacc.2016.07.794. J Am Coll Cardiol. 2017. PMID: 28183515 No abstract available.
-
LDLR Variant Databases and Familial Hypercholesterolemia Population Studies.J Am Coll Cardiol. 2017 Feb 14;69(6):754-755. doi: 10.1016/j.jacc.2016.09.988. J Am Coll Cardiol. 2017. PMID: 28183516 No abstract available.
-
Reply: Familial Hypercholesterolemia: Independent Malignity and LDLR Variant Databases.J Am Coll Cardiol. 2017 Feb 14;69(6):755. doi: 10.1016/j.jacc.2016.10.079. J Am Coll Cardiol. 2017. PMID: 28183517 No abstract available.
References
-
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. - PubMed
-
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. - PubMed
-
- Gidding SS, Ann Champagne M, de Ferranti SD, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015 Dec 1;132(22):2167–92. - PubMed
-
- Fouchier SW, Defesche JC, Umans-Eckenhausen MW, Kastelein JP. The molecular basis of familial hypercholesterolemia in The Netherlands. Hum Genet. 2001;109(6):602–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UC2 HL103010/HL/NHLBI NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- U54 HG003273/HG/NHGRI NIH HHS/United States
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- R01 HL105756/HL/NHLBI NIH HHS/United States
- K01 HL125751/HL/NHLBI NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- RC2 HL102419/HL/NHLBI NIH HHS/United States
- R01 HL103612/HL/NHLBI NIH HHS/United States
- RC2 HL102923/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- N01 HC085080/HL/NHLBI NIH HHS/United States
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- R01 HL120393/HL/NHLBI NIH HHS/United States
- U54 HG003067/HG/NHGRI NIH HHS/United States
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- N02 HL064278/HL/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- N01 HC085082/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- HHSN268200800007C/HL/NHLBI NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- N01 HC085083/HL/NHLBI NIH HHS/United States
- HHSN268201300048C/HL/NHLBI NIH HHS/United States
- R01 HL087652/HL/NHLBI NIH HHS/United States
- R01 HL127564/HL/NHLBI NIH HHS/United States
- HHSN268201300049C/HL/NHLBI NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- HHSN268201300047C/HL/NHLBI NIH HHS/United States
- HHSN268201300050C/HL/NHLBI NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- HHSN268201300046C/HL/NHLBI NIH HHS/United States
- N01 HC085081/HL/NHLBI NIH HHS/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

