Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury
- PMID: 27050417
- PMCID: PMC5105355
- DOI: 10.1089/neu.2015.4348
Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury
Abstract
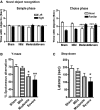
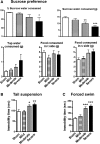
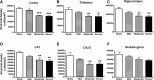
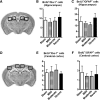
Clinical and experimental studies show that spinal cord injury (SCI) can cause cognitive impairment and depression that can significantly impact outcomes. Thus, identifying mechanisms responsible for these less well-examined, important SCI consequences may provide targets for more effective therapeutic intervention. To determine whether cognitive and depressive-like changes correlate with injury severity, we exposed mice to sham, mild, moderate, or severe SCI using the Infinite Horizon Spinal Cord Impactor and evaluated performance on a variety of neurobehavioral tests that are less dependent on locomotion. Cognitive impairment in Y-maze, novel objective recognition, and step-down fear conditioning tasks were increased in moderate- and severe-injury mice that also displayed depressive-like behavior as quantified in the sucrose preference, tail suspension, and forced swim tests. Bromo-deoxyuridine incorporation with immunohistochemistry revealed that SCI led to a long-term reduction in the number of newly-generated immature neurons in the hippocampal dentate gyrus, accompanied by evidence of greater neuronal endoplasmic reticulum (ER) stress. Stereological analysis demonstrated that moderate/severe SCI reduced neuronal survival and increased the number of activated microglia chronically in the cerebral cortex and hippocampus. The potent microglial activator cysteine-cysteine chemokine ligand 21 (CCL21) was elevated in the brain sites after SCI in association with increased microglial activation. These findings indicate that SCI causes chronic neuroinflammation that contributes to neuronal loss, impaired hippocampal neurogenesis and increased neuronal ER stress in important brain regions associated with cognitive decline and physiological depression. Accumulation of CCL21 in brain may subserve a pathophysiological role in cognitive changes and depression after SCI.
Keywords: ER stress; adult neurogenesis; brain; cognition/depression; spinal cord injury.
Conflict of interest statement
Author Disclosure Statement No competing financial interests exist.
Figures





Similar articles
-
Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways.J Neurosci. 2014 Aug 13;34(33):10989-1006. doi: 10.1523/JNEUROSCI.5110-13.2014. J Neurosci. 2014. PMID: 25122899 Free PMC article.
-
Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation.Cell Cycle. 2014;13(15):2446-58. doi: 10.4161/cc.29420. Cell Cycle. 2014. PMID: 25483194 Free PMC article.
-
Spinal Cord Injury Impairs Neurogenesis and Induces Glial Reactivity in the Hippocampus.Neurochem Res. 2017 Aug;42(8):2178-2190. doi: 10.1007/s11064-017-2225-9. Epub 2017 Mar 13. Neurochem Res. 2017. PMID: 28290135
-
Dementia, Depression, and Associated Brain Inflammatory Mechanisms after Spinal Cord Injury.Cells. 2020 Jun 8;9(6):1420. doi: 10.3390/cells9061420. Cells. 2020. PMID: 32521597 Free PMC article. Review.
-
Multidimensional review of cognitive impairment after spinal cord injury.Acta Neurol Belg. 2021 Feb;121(1):37-46. doi: 10.1007/s13760-020-01507-y. Epub 2020 Sep 28. Acta Neurol Belg. 2021. PMID: 32989706 Review.
Cited by
-
Role of endoplasmic reticulum stress in depression (Review).Mol Med Rep. 2019 Dec;20(6):4774-4780. doi: 10.3892/mmr.2019.10789. Epub 2019 Oct 31. Mol Med Rep. 2019. PMID: 31702816 Free PMC article. Review.
-
Intersection of hippocampus and spinal cord: a focus on the hippocampal alpha-synuclein accumulation, dopaminergic receptors, neurogenesis, and cognitive function following spinal cord injury in male rats.BMC Neurosci. 2022 Jul 12;23(1):44. doi: 10.1186/s12868-022-00729-5. BMC Neurosci. 2022. PMID: 35820831 Free PMC article.
-
The course and prognostic factors of cognitive status after central nervous system trauma: a systematic review protocol.BMJ Open. 2017 Sep 18;7(9):e017165. doi: 10.1136/bmjopen-2017-017165. BMJ Open. 2017. PMID: 28928193 Free PMC article.
-
Cellular Response to Unfolded Proteins in Depression.Life (Basel). 2021 Dec 10;11(12):1376. doi: 10.3390/life11121376. Life (Basel). 2021. PMID: 34947907 Free PMC article. Review.
-
Neuromuscular electrical stimulation to combat cognitive aging in people with spinal cord injury: protocol for a single case experimental design study.BMC Neurol. 2024 Jun 11;24(1):197. doi: 10.1186/s12883-024-03699-9. BMC Neurol. 2024. PMID: 38862912 Free PMC article.
References
-
- Davidoff G.N., Roth E.J., and Richards J.S. (1992). Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch. Phys. Med. Rehabil. 73, 275–284 - PubMed
-
- Lazzaro I., Tran Y., Wijesuriya N., and Craig A. (2013). Central correlates of impaired information processing in people with spinal cord injury. J. Clin. Neurophysiol. 30, 59–65 - PubMed
-
- Dowler R.N., Harrington D.L., Haaland K.Y., Swanda R.M., Fee F., and Fiedler K. (1997). Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J. Int. Neuropsychol. Soc. 3, 464–472 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

