Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons
- PMID: 27050422
- PMCID: PMC4822954
- DOI: 10.1371/journal.pone.0152371
Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons
Abstract
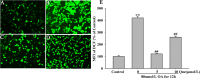
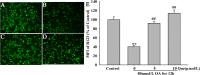
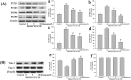
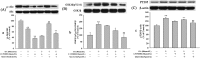
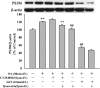
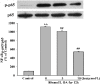
Increasing evidence shows that oxidative stress and the hyperphosphorylation of tau protein play essential roles in the progression of Alzheimer's disease (AD). Quercetin is a major flavonoid that has anti-oxidant, anti-cancer and anti-inflammatory properties. We investigated the neuroprotective effects of quercetin to HT22 cells (a cell line from mouse hippocampal neurons). We found that Okadaic acid (OA) induced the hyperphosphorylation of tau protein at Ser199, Ser396, Thr205, and Thr231 and produced oxidative stress to the HT22 cells. The oxidative stress suppressed the cell viability and decreased the levels of lactate dehydrogenase (LDH), superoxide dismutase (SOD), mitochondria membrane potential (MMP) and Glutathione peroxidase (GSH-Px). It up-regulated malondialdehyde (MDA) production and intracellular reactive oxygen species (ROS). In addition, phosphoinositide 3 kinase/protein kinase B/Glycogen synthase kinase3β (PI3K/Akt/GSK3β) and mitogen activated protein kinase (MAPK) were also involved in this process. We found that pre-treatment with quercetin can inhibited OA-induced the hyperphosphorylation of tau protein and oxidative stress. Moreover, pre-treatment with quercetin not only inhibited OA-induced apoptosis via the reduction of Bax, and up-regulation of cleaved caspase 3, but also via the inhibition of PI3K/Akt/GSK3β, MAPKs and activation of NF-κB p65. Our findings suggest the therapeutic potential of quercetin to treat AD.
Conflict of interest statement
Figures







Similar articles
-
Jatrorrhizine Protects Against Okadaic Acid Induced Oxidative Toxicity Through Inhibiting the Mitogen-Activated Protein Kinases Pathways in HT22 Hippocampal Neurons.CNS Neurol Disord Drug Targets. 2015;14(10):1334-42. doi: 10.2174/1871527314666150821104455. CNS Neurol Disord Drug Targets. 2015. PMID: 26295822
-
Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells.Neurosci Lett. 2013 Feb 22;535:146-51. doi: 10.1016/j.neulet.2012.12.051. Epub 2013 Jan 8. Neurosci Lett. 2013. PMID: 23313590
-
Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation.J Alzheimers Dis. 2014;39(2):331-46. doi: 10.3233/JAD-131457. J Alzheimers Dis. 2014. PMID: 24164737
-
A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells.Int J Mol Sci. 2022 Oct 4;23(19):11746. doi: 10.3390/ijms231911746. Int J Mol Sci. 2022. PMID: 36233051 Free PMC article. Review.
-
Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism.Front Immunol. 2022 Jul 22;13:943321. doi: 10.3389/fimmu.2022.943321. eCollection 2022. Front Immunol. 2022. PMID: 35935939 Free PMC article. Review.
Cited by
-
Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer's Disease: Dietary Plant Polyphenols.Int J Mol Sci. 2022 Nov 11;23(22):13886. doi: 10.3390/ijms232213886. Int J Mol Sci. 2022. PMID: 36430365 Free PMC article. Review.
-
High-throughput screening for amyloid-β binding natural small-molecules based on the combinational use of biolayer interferometry and UHPLC-DAD-Q/TOF-MS/MS.Acta Pharm Sin B. 2022 Apr;12(4):1723-1739. doi: 10.1016/j.apsb.2021.08.030. Epub 2021 Sep 4. Acta Pharm Sin B. 2022. PMID: 35847494 Free PMC article.
-
Molecular Signaling Pathways of Quercetin in Alzheimer's Disease: A Promising Arena.Cell Mol Neurobiol. 2024 Dec 24;45(1):8. doi: 10.1007/s10571-024-01526-w. Cell Mol Neurobiol. 2024. PMID: 39719518 Free PMC article. Review.
-
A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants.Int J Mol Sci. 2020 Oct 21;21(20):7774. doi: 10.3390/ijms21207774. Int J Mol Sci. 2020. PMID: 33096625 Free PMC article. Review.
-
The Anti-Inflammatory Effects of Blueberries in an Animal Model of Post-Traumatic Stress Disorder (PTSD).PLoS One. 2016 Sep 7;11(9):e0160923. doi: 10.1371/journal.pone.0160923. eCollection 2016. PLoS One. 2016. PMID: 27603014 Free PMC article.
References
-
- Thal DR, von Arnim C, Griffin WS, Yamaguchi H, Mrak RE, Attems J, et al. Pathology of clinical and preclinical Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 2013. November;263 Suppl 2:S137–45. - PubMed
-
- Zimmer ER, Kalinine E, Haas CB, Torrez VR, Souza DO, Muller AP, et al. Pretreatment with memantine prevents Alzheimer-like alterations induced by intrahippocampal okadaic acid administration in rats. Curr Alzheimer Res. 2012. December;9(10):1182–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

