Integrated Regulation of Hepatic Lipid and Glucose Metabolism by Adipose Triacylglycerol Lipase and FoxO Proteins
- PMID: 27050511
- PMCID: PMC5346032
- DOI: 10.1016/j.celrep.2016.03.021
Integrated Regulation of Hepatic Lipid and Glucose Metabolism by Adipose Triacylglycerol Lipase and FoxO Proteins
Abstract
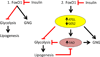
Metabolism is a highly integrated process that is coordinately regulated between tissues and within individual cells. FoxO proteins are major targets of insulin action and contribute to the regulation of gluconeogenesis, glycolysis, and lipogenesis in the liver. However, the mechanisms by which FoxO proteins exert these diverse effects in an integrated fashion remain poorly understood. We report that FoxO proteins also exert important effects on intrahepatic lipolysis and fatty acid oxidation via the regulation of adipose triacylglycerol lipase (ATGL), which mediates the first step in lipolysis, and its inhibitor, the G0/S1 switch 2 gene (G0S2). We also find that ATGL-dependent lipolysis plays a critical role in mediating diverse effects of FoxO proteins in the liver, including effects on gluconeogenic, glycolytic, and lipogenic gene expression and metabolism. These results indicate that intrahepatic lipolysis plays a critical role in mediating and integrating the regulation of glucose and lipid metabolism downstream of FoxO proteins.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







Similar articles
-
G0S2: A small giant controller of lipolysis and adipose-liver fatty acid flux.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt B):1146-1154. doi: 10.1016/j.bbalip.2017.06.007. Epub 2017 Jun 21. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28645852 Free PMC article. Review.
-
Regulation of Hepatic Glucose Metabolism by FoxO Proteins, an Integrated Approach.Curr Top Dev Biol. 2018;127:119-147. doi: 10.1016/bs.ctdb.2017.10.005. Epub 2017 Dec 19. Curr Top Dev Biol. 2018. PMID: 29433735 Review.
-
The role of adipose triglyceride lipase in lipid and glucose homeostasis: lessons from transgenic mice.Lipids Health Dis. 2019 Nov 22;18(1):204. doi: 10.1186/s12944-019-1151-z. Lipids Health Dis. 2019. PMID: 31757217 Free PMC article. Review.
-
FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression.J Biol Chem. 2006 Apr 14;281(15):10105-17. doi: 10.1074/jbc.M600272200. Epub 2006 Feb 21. J Biol Chem. 2006. PMID: 16492665
-
TFE3 controls lipid metabolism in adipose tissue of male mice by suppressing lipolysis and thermogenesis.Endocrinology. 2013 Oct;154(10):3577-88. doi: 10.1210/en.2013-1203. Epub 2013 Jul 24. Endocrinology. 2013. PMID: 23885019
Cited by
-
Integrated Analyses Identify Key Molecules and Reveal the Potential Mechanism of miR-182-5p/FOXO1 Axis in Alcoholic Liver Disease.Front Med (Lausanne). 2021 Dec 7;8:767584. doi: 10.3389/fmed.2021.767584. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34950682 Free PMC article.
-
The effect of G0S2 on insulin sensitivity: A proteomic analysis in a G0S2-overexpressed high-fat diet mouse model.Front Endocrinol (Lausanne). 2023 Mar 23;14:1130350. doi: 10.3389/fendo.2023.1130350. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37033250 Free PMC article.
-
Evaluating the roles of microRNAs associated with nonalcoholic fatty liver disease in hepatocellular carcinoma tumorigenesis: a systematic review and network analysis.Front Med (Lausanne). 2024 Nov 15;11:1462513. doi: 10.3389/fmed.2024.1462513. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39618816 Free PMC article.
-
Recent advances on the role of G0S2.Discov Oncol. 2025 Jul 18;16(1):1362. doi: 10.1007/s12672-025-03198-4. Discov Oncol. 2025. PMID: 40679675 Free PMC article. Review.
-
Fatty liver diseases, mechanisms, and potential therapeutic plant medicines.Chin J Nat Med. 2020 Mar;18(3):161-168. doi: 10.1016/S1875-5364(20)30017-0. Chin J Nat Med. 2020. PMID: 32245585 Free PMC article. Review.
References
-
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature medicine. 2004;10:268–274. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

