Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System
- PMID: 27050519
- PMCID: PMC5050004
- DOI: 10.1016/j.celrep.2016.03.028
Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System
Abstract
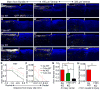
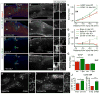
How aging impacts axon regeneration after CNS injury is not known. We assessed the impact of age on axon regeneration induced by Pten deletion in corticospinal and rubrospinal neurons, two neuronal populations with distinct innate regenerative abilities. As in young mice, Pten deletion in older mice remains effective in preventing axotomy-induced decline in neuron-intrinsic growth state, as assessed by mTOR activity, neuronal soma size, and axonal growth proximal to a spinal cord injury. However, axonal regeneration distal to injury is greatly diminished, accompanied by increased expression of astroglial and inflammatory markers at the injury site. Thus, the mammalian CNS undergoes an age-dependent decline in axon regeneration, as revealed when neuron-intrinsic growth state is elevated. These results have important implications for developing strategies to promote axonal repair after CNS injuries or diseases, which increasingly affect middle-aged to aging populations.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. - PubMed
-
- Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24:577–583. - PubMed
-
- Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. - PubMed
-
- Bradke F, Marin O. Editorial overview: development and regeneration: nervous system development and regeneration. Curr Opin Neurobiol. 2014;27:iv–vi. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

