Mitigation of reversible self-association and viscosity in a human IgG1 monoclonal antibody by rational, structure-guided Fv engineering
- PMID: 27050875
- PMCID: PMC4968137
- DOI: 10.1080/19420862.2016.1171444
Mitigation of reversible self-association and viscosity in a human IgG1 monoclonal antibody by rational, structure-guided Fv engineering
Abstract
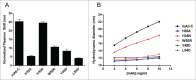
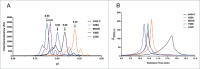
Undesired solution behaviors such as reversible self-association (RSA), high viscosity, and liquid-liquid phase separation can introduce substantial challenges during development of monoclonal antibody formulations. Although a global mechanistic understanding of RSA (i.e., native and reversible protein-protein interactions) is sufficient to develop robust formulation controls, its mitigation via protein engineering requires knowledge of the sites of protein-protein interactions. In the study reported here, we coupled our previous hydrogen-deuterium exchange mass spectrometry findings with structural modeling and in vitro screening to identify the residues responsible for RSA of a model IgG1 monoclonal antibody (mAb-C), and rationally engineered variants with improved solution properties (i.e., reduced RSA and viscosity). Our data show that mutation of either solvent-exposed aromatic residues within the heavy and light chain variable regions or buried residues within the heavy chain/light chain interface can significantly mitigate RSA and viscosity by reducing the IgG's surface hydrophobicity. The engineering strategy described here highlights the utility of integrating complementary experimental and in silico methods to identify mutations that can improve developability, in particular, high concentration solution properties, of candidate therapeutic antibodies.
Keywords: AC-SINS; Antibody engineering; DLS; antibody developability; homology modeling; monoclonal antibody; reversible self-association; viscosity.
Figures



References
-
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767-74; PMID:20811384; http://dx.doi.org/10.1038/nrd3229 - DOI - PubMed
-
- Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci 2007; 96:1-26; PMID:16998873; http://dx.doi.org/10.1002/jps.20727 - DOI - PubMed
-
- Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci 2005; 94:1928-40; PMID:16052543; http://dx.doi.org/10.1002/jps.20347 - DOI - PubMed
-
- Mason BD, Zhang L, Remmele RL Jr, Zhang J. Opalescence of an IgG2 monoclonal antibody solution as it relates to liquid-liquid phase separation. J Pharm Sci 2011; 100:4587-96; PMID:21638285; http://dx.doi.org/10.1002/jps.22650 - DOI - PubMed
-
- Nishi H, Miyajima M, Nakagami H, Noda M, Uchiyama S, Fukui K. Phase separation of an IgG1 antibody solution under a low ionic strength condition. Pharm Res 2010; 27:1348-60; PMID:20401522; http://dx.doi.org/10.1007/s11095-010-0125-7 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
