Glycogen metabolism in humans
- PMID: 27051594
- PMCID: PMC4802397
- DOI: 10.1016/j.bbacli.2016.02.001
Glycogen metabolism in humans
Abstract
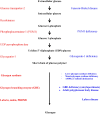
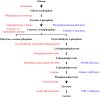
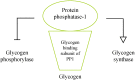
In the human body, glycogen is a branched polymer of glucose stored mainly in the liver and the skeletal muscle that supplies glucose to the blood stream during fasting periods and to the muscle cells during muscle contraction. Glycogen has been identified in other tissues such as brain, heart, kidney, adipose tissue, and erythrocytes, but glycogen function in these tissues is mostly unknown. Glycogen synthesis requires a series of reactions that include glucose entrance into the cell through transporters, phosphorylation of glucose to glucose 6-phosphate, isomerization to glucose 1-phosphate, and formation of uridine 5'-diphosphate-glucose, which is the direct glucose donor for glycogen synthesis. Glycogenin catalyzes the formation of a short glucose polymer that is extended by the action of glycogen synthase. Glycogen branching enzyme introduces branch points in the glycogen particle at even intervals. Laforin and malin are proteins involved in glycogen assembly but their specific function remains elusive in humans. Glycogen is accumulated in the liver primarily during the postprandial period and in the skeletal muscle predominantly after exercise. In the cytosol, glycogen breakdown or glycogenolysis is carried out by two enzymes, glycogen phosphorylase which releases glucose 1-phosphate from the linear chains of glycogen, and glycogen debranching enzyme which untangles the branch points. In the lysosomes, glycogen degradation is catalyzed by α-glucosidase. The glucose 6-phosphatase system catalyzes the dephosphorylation of glucose 6-phosphate to glucose, a necessary step for free glucose to leave the cell. Mutations in the genes encoding the enzymes involved in glycogen metabolism cause glycogen storage diseases.
Keywords: Glucokinase; Glucose; Glycogen phosphorylase; Glycogen storage diseases; Glycogen synthase; Phosphoglucomutases; α-Glucosidase.
Figures

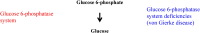
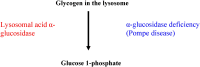
References
-
- De Giorgis V., Veggiotti P. GLUT1 deficiency syndrome 2013: current state of the art. Seizure. 2013;22(10):803–811. - PubMed
-
- Scholl-Bürgi S., Höller A., Pichler K., Michel M., Haberlandt E., Karall D. Ketogenic diets in patients with inherited metabolic disorders. J. Inherit. Metab. Dis. 2015;38(4):765–773. - PubMed
-
- Gottesman I., Mandarino L., Gerich J. Estimation and kinetic analysis of insulin-independent glucose uptake in human subjects. Am. J. Physiol. 1983;244(6):E632–E635. - PubMed
-
- Lauritzen H.P., Schertzer J.D. Measuring GLUT4 translocation in mature muscle fibers. Am. J. Physiol. Endocrinol. Metab. 2010;299(2):E169–E179. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

