RXR controlled regulatory networks identified in mouse brain counteract deleterious effects of Aβ oligomers
- PMID: 27051978
- PMCID: PMC4823697
- DOI: 10.1038/srep24048
RXR controlled regulatory networks identified in mouse brain counteract deleterious effects of Aβ oligomers
Abstract
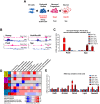
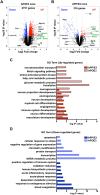
Bexarotene, a selective agonist for Retinoid X receptors (RXR) improves cognitive deficits and amyloid-β (Aβ) clearance in mice. Here we examine if the effect of bexarotene on RXR cistrome and transcriptomes depend on APOE isoform and Aβ deposition. We found bexarotene increased RXR binding to promoter regions in cortex of APOE3 mice. Rho family GTPases and Wnt signaling pathway were highly enriched in ChIP-seq and RNA-seq datasets and members of those pathways - Lrp1, Lrp5, Sfrp5 and Sema3f were validated. The effect of APOE isoform was compared in APOE3 and APOE4 mice and we found significant overlapping in affected pathways. ChIP-seq using mouse embryonic stem cells and enrichment levels of histone marks H3K4me3 and H3K27me3 revealed that, bexarotene induced epigenetic changes, consistent with increased neuronal differentiation and in correlation with changes in transcription. Comparison of transcriptome in APOE3 and APP/APOE3 mice revealed that amyloid deposition significantly affects the response to bexarotene. In primary neurons, bexarotene ameliorated the damaged dendrite complexity and loss of neurites caused by Aβ42. Finally, we show that the disruption of actin cytoskeleton induced by Aβ42 in vitro was inhibited by bexarotene treatment. Our results suggest a mechanism to establish RXR therapeutic targets with significance in neurodegeneration.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Bexarotene-Activated Retinoid X Receptors Regulate Neuronal Differentiation and Dendritic Complexity.J Neurosci. 2015 Aug 26;35(34):11862-76. doi: 10.1523/JNEUROSCI.1001-15.2015. J Neurosci. 2015. PMID: 26311769 Free PMC article.
-
RNA-sequencing reveals transcriptional up-regulation of Trem2 in response to bexarotene treatment.Neurobiol Dis. 2015 Oct;82:132-140. doi: 10.1016/j.nbd.2015.05.019. Epub 2015 Jun 10. Neurobiol Dis. 2015. PMID: 26071899 Free PMC article.
-
Amyloid-β pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo.J Biol Chem. 2014 Oct 31;289(44):30538-30555. doi: 10.1074/jbc.M114.600833. Epub 2014 Sep 12. J Biol Chem. 2014. PMID: 25217640 Free PMC article.
-
The novel function of bexarotene for neurological diseases.Ageing Res Rev. 2023 Sep;90:102021. doi: 10.1016/j.arr.2023.102021. Epub 2023 Jul 24. Ageing Res Rev. 2023. PMID: 37495118 Review.
-
Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology.Microsc Res Tech. 2000 Aug 15;50(4):278-81. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10936880 Review.
Cited by
-
Proteomic Profiling of Extracellular Vesicles Isolated From Cerebrospinal Fluid of Former National Football League Players at Risk for Chronic Traumatic Encephalopathy.Front Neurosci. 2019 Oct 9;13:1059. doi: 10.3389/fnins.2019.01059. eCollection 2019. Front Neurosci. 2019. PMID: 31649498 Free PMC article.
-
Age-Associated DNA Methylation Patterns Are Shared Between the Hippocampus and Peripheral Blood Cells.Front Genet. 2020 Mar 6;11:111. doi: 10.3389/fgene.2020.00111. eCollection 2020. Front Genet. 2020. PMID: 32211019 Free PMC article.
-
Therapeutic potential of nuclear receptor agonists in Alzheimer's disease.J Lipid Res. 2017 Oct;58(10):1937-1949. doi: 10.1194/jlr.R075556. Epub 2017 Mar 6. J Lipid Res. 2017. PMID: 28264880 Free PMC article. Review.
-
The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer's Disease and Other Neurodegenerative Disorders.Neurochem Res. 2020 May;45(5):972-988. doi: 10.1007/s11064-020-02993-5. Epub 2020 Mar 13. Neurochem Res. 2020. PMID: 32170673 Free PMC article. Review.
-
Alzheimer's Disease, a Lipid Story: Involvement of Peroxisome Proliferator-Activated Receptor α.Cells. 2020 May 14;9(5):1215. doi: 10.3390/cells9051215. Cells. 2020. PMID: 32422896 Free PMC article. Review.
References
-
- Koldamova R. P. et al. The liver X receptor ligand T0901317 decreases Amyloid-β production in vitro and in a mouse model of Alzheimer’s Disease. J Biol Chem 280, 4079–4088 (2005). - PubMed
-
- Koldamova R., Staufenbiel M. & Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem 280, 43224–43235 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

