Glucansucrase Gtf180-ΔN of Lactobacillus reuteri 180: enzyme and reaction engineering for improved glycosylation of non-carbohydrate molecules
- PMID: 27052379
- PMCID: PMC4980424
- DOI: 10.1007/s00253-016-7476-x
Glucansucrase Gtf180-ΔN of Lactobacillus reuteri 180: enzyme and reaction engineering for improved glycosylation of non-carbohydrate molecules
Abstract
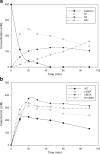
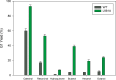
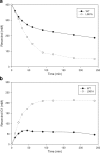
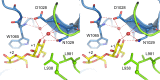
Glucansucrases have a broad acceptor substrate specificity and receive increased attention as biocatalysts for the glycosylation of small non-carbohydrate molecules using sucrose as donor substrate. However, the main glucansucrase-catalyzed reaction results in synthesis of α-glucan polysaccharides from sucrose, and this strongly impedes the efficient glycosylation of non-carbohydrate molecules and complicates downstream processing of glucosylated products. This paper reports that suppressing α-glucan synthesis by mutational engineering of the Gtf180-ΔN enzyme of Lactobacillus reuteri 180 results in the construction of more efficient glycosylation biocatalysts. Gtf180-ΔN mutants (L938F, L981A, and N1029M) with an impaired α-glucan synthesis displayed a substantial increase in monoglycosylation yields for several phenolic and alcoholic compounds. Kinetic analysis revealed that these mutants possess a higher affinity for the model acceptor substrate catechol but a lower affinity for its mono-α-D-glucoside product, explaining the improved monoglycosylation yields. Analysis of the available high resolution 3D crystal structure of the Gtf180-ΔN protein provided a clear understanding of how mutagenesis of residues L938, L981, and N1029 impaired α-glucan synthesis, thus yielding mutants with an improved glycosylation potential.
Keywords: Acceptor reaction; Catechol; Enzyme engineering; Glucansucrase; Glycosylation; Lactobacillus reuteri.
Figures

References
-
- Auriol D, Nalin R, Robe P, Lefevre F (2012) Phenolic compounds with cosmetic and therapeutic applications. EP2027279.
-
- De Winter K, Cerdobbel A, Soetaert W, Desmet T. Operational stability of immobilized sucrose phosphorylase: continuous production of α-glucose-1-phosphate at elevated temperatures. Process Biochem. 2011;46:2074–2078. doi: 10.1016/j.procbio.2011.08.002. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

