Stability of Protein-Specific Hydration Shell on Crowding
- PMID: 27052457
- PMCID: PMC7849722
- DOI: 10.1021/jacs.6b01989
Stability of Protein-Specific Hydration Shell on Crowding
Abstract
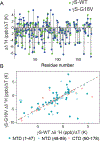
We demonstrate that the effect of protein crowding is critically dependent on the stability of the protein's hydration shell, which can dramatically vary between different proteins. In the human eye lens, γS-crystallin (γS-WT) forms a densely packed transparent hydrogel with a high refractive index, making it an ideal system for studying the effects of protein crowding. A single point mutation generates the cataract-related variant γS-G18V, dramatically altering the optical properties of the eye lens. This system offers an opportunity to explore fundamental questions regarding the effect of protein crowding, using γS-WT and γS-G18V: (i) how do the diffusion dynamics of hydration water change as a function of protein crowding?; and (ii) upon hydrogel formation of γS-WT, has a dynamic transition occurred generating a single population of hydration water, or do populations of bulk and hydration water coexist? Using localized spin probes, we separately probe the local translational diffusivity of both surface hydration and interstitial water of γS-WT and γS-G18V in solution. Surprisingly, we find that under the influence of hydrogel formation at highly crowded γS-WT concentrations up to 500 mg/mL, the protein hydration shell remains remarkably dynamic, slowing by less than a factor of 2, if at all, compared to that in dilute protein solutions of ∼5 mg/mL. Upon self-crowding, the population of this robust surface hydration water increases, while a significant bulk-like water population coexists even at ∼500 mg/mL protein concentrations. In contrast, surface water of γS-G18V irreversibly dehydrates with moderate concentration increases or subtle alterations to the solution conditions, demonstrating that the effect of protein crowding is highly dependent on the stability of the protein-specific hydration shell. The core function of γS-crystallin in the eye lens may be precisely its capacity to preserve a robust hydration shell, whose stability is abolished by a single G18V mutation.
Figures




References
-
- Minton AP Biopolymers 1981, 20, 2093.
-
- Miklos AC; Sarkar M; Wang Y; Pielak GJ Journal of the American Chemical Society 2011, 133, 7116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

