Reduced retinal transduction and enhanced transgene-directed immunogenicity with intravitreal delivery of rAAV following posterior vitrectomy in dogs
- PMID: 27052802
- PMCID: PMC4891289
- DOI: 10.1038/gt.2016.31
Reduced retinal transduction and enhanced transgene-directed immunogenicity with intravitreal delivery of rAAV following posterior vitrectomy in dogs
Abstract
Adeno-associated virus (AAV) vector-based gene therapy is a promising treatment strategy for delivery of neurotrophic transgenes to retinal ganglion cells (RGCs) in glaucoma patients. Retinal distribution of transgene expression following intravitreal injection (IVT) of AAV is variable in animal models and the vitreous humor may represent a barrier to initial vector penetration. The primary goal of our study was to investigate the effect of prior core vitrectomy with posterior hyaloid membrane peeling on pattern and efficiency of transduction of a capsid amino acid substituted AAV2 vector, carrying the green fluorescent protein (GFP) reporter transgene following IVT in dogs. When progressive intraocular inflammation developed starting 4 weeks post IVT, the study plan was modified to allow detailed characterization of the etiology as a secondary goal. Unexpectedly, surgical vitrectomy was found to significantly limit transduction, whereas in non-vitrectomized eyes transduction efficiency reached upwards to 37.3% of RGC layer cells. The developing retinitis was characterized by mononuclear cell infiltrates resulting from a delayed-type hypersensitivity reaction, which we suspect was directed at the GFP transgene. Our results, in a canine large animal model, support caution when considering surgical vitrectomy before IVT for retinal gene therapy in patients, as prior vitrectomy appears to significantly reduce transduction efficiency and may predispose the patient to development of vector-induced immune reactions.
Conflict of interest statement
WWH and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. The remaining authors declare no conflict of interest.
Figures
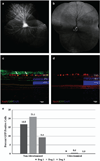

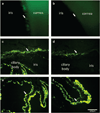
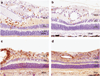
Comment on
-
In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous.Sci Transl Med. 2013 Jun 12;5(189):189ra76. doi: 10.1126/scitranslmed.3005708. Sci Transl Med. 2013. PMID: 23761039
References
-
- Chader GJ. Advances in glaucoma treatment and management: neurotrophic agents. Invest Ophthalmol Vis Sci. 2012;53:2501–2505. - PubMed
-
- Hellstrom M, Pollett MA, Harvey AR. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J Neurotrauma. 2011;28:2475–2483. - PubMed
-
- van Adel BA, Kostic C, Deglon N, Ball AK, Arsenijevic Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–115. - PubMed
-
- Hellstrom M, Harvey AR. Retinal ganglion cell gene therapy and visual system repair. Curr Gene Ther. 2011;11:116–131. - PubMed
-
- Harvey AR, Hellström M, Rodger J. Gene therapy and transplantation in the retinofugal pathway. Prog Brain Res. 2009;175:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

