Population Pharmacokinetics of Oral Topotecan in Infants and Very Young Children with Brain Tumors Demonstrates a Role of ABCG2 rs4148157 on the Absorption Rate Constant
- PMID: 27052877
- PMCID: PMC4931885
- DOI: 10.1124/dmd.115.068676
Population Pharmacokinetics of Oral Topotecan in Infants and Very Young Children with Brain Tumors Demonstrates a Role of ABCG2 rs4148157 on the Absorption Rate Constant
Abstract
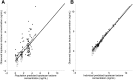
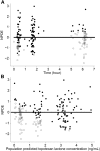
For infants and very young children with brain tumors, chemotherapy after surgical resection is the main treatment due to neurologic and neuroendocrine adverse effects from whole brain irradiation. Topotecan, an anticancer drug with antitumor activity against pediatric brain tumors, can be given intravenous or orally. However, high interpatient variability in oral drug bioavailability is common in children less than 3 years old. Therefore, this study aimed to determine the population pharmacokinetics of oral topotecan in infants and very young children, specifically evaluating the effects of age and ABCG2 and ABCB1 on the absorption rate constant (Ka), as well as other covariate effects on all pharmacokinetic parameters. A nonlinear mixed effects model was implemented in Monolix 4.3.2 (Lixoft, Orsay, France). A one-compartment model with first-order input and first-order elimination was found to adequately characterize topotecan lactone concentrations with population estimates as [mean (S.E.)]; Ka = 0.61 (0.11) h(-1), apparent volume of distribution (V/F) = 40.2 (7.0) l, and apparent clearance (CL/F) = 40.0 (2.9) l/h. After including the body surface area in the V/F and CL/F as a power model centered on the population median, the ABCG2 rs4148157 allele was found to play a significant role in the value of Ka Patients homozygous or heterozygous for G>A demonstrated a Ka value 2-fold higher than their GG counterparts, complemented with a 2-fold higher maximal concentration as well. These results demonstrate a possible role for the ABCG2 rs4148157 allele in the pharmacokinetics of oral topotecan in infants and very young children, and warrants further investigation.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Alcorn J, McNamara PJ. (2003) Pharmacokinetics in the newborn. Adv Drug Deliv Rev 55:667–686. - PubMed
-
- Anderson BJ, Allegaert K, Holford NH. (2006) Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165:819–829. - PubMed
-
- Beijnen JH, Smith BR, Keijer WJ, van Gijn R, ten Bokkel Huinink WW, Vlasveld LT, Rodenhuis S, Underberg WJM. (1990) High-performance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. J Pharm Biomed Anal 8:789–794. - PubMed
-
- Berseth CL. (1996) Gastrointestinal motility in the neonate. Clin Perinatol 23:179–190. - PubMed
-
- Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE. (1999) Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res 59:5938–5946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

