Characterization of Atomoxetine Biotransformation and Implications for Development of PBPK Models for Dose Individualization in Children
- PMID: 27052878
- PMCID: PMC4931890
- DOI: 10.1124/dmd.116.069518
Characterization of Atomoxetine Biotransformation and Implications for Development of PBPK Models for Dose Individualization in Children
Abstract
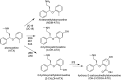
Atomoxetine (ATX) is a second-line nonstimulant medication used to control symptoms of attention deficit hyperactivity disorder (ADHD). Inconsistent therapeutic efficacy has been reported with ATX, which may be related to variable CYP2D6-mediated drug clearance. We characterized ATX metabolism in a panel of human liver samples as a basis for a bottom-up PBPK model to aid in ATX exposure prediction and control. Km, Vmax, and Clint values in pooled human liver microsomes (HLMs) were 2.4 µM, 479 pmol/min/mg protein, and 202 µl/min/mg protein, respectively. Mean population values of kinetic parameters are not adequate to describe variability in a population, given that Km, Vmax, and Clint values from single-donor HLMs ranged from 0.93 to 79.2 µM, 20.0 to 1600 pmol/min/mg protein, and 0.3 to 936 µl/min/mg protein. All kinetic parameters were calculated from 4-hydroxyatomoxetine (4-OH-ATX) formation. CYP2E1 and CYP3A contributed to 4-OH-ATX formation in livers with CYP2D6 intermediate and poor metabolizer status. In HLMs with lower CYP2D6 activity levels, 2-hydroxymethylatomoxetine (2-CH2OH-ATX) formation became a more predominant pathway of metabolism, which appeared to be catalyzed by CYP2B6. ATX biotransformation at clinically relevant plasma concentrations was characterized in a panel of pediatric HLM (n = 116) samples by evaluating primary metabolites. Competing pathways of ATX metabolism [N-desmethylatomoxetine (NDM-ATX) and 2-CH2OH-ATX formation] had increasing importance in livers with lesser CYP2D6 activity, but, overall ATX clearance was still compromised. Modeling ATX exposure to individualize therapy would require comprehensive knowledge of factors that affect CYP2D6 activity as well as an understanding of competing pathways, particularly for individuals with lower CYP2D6 activity.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures






References
-
- Asherson P, Akehurst R, Kooij JJ, Huss M, Beusterien K, Sasané R, Gholizadeh S, Hodgkins P. (2012) Under diagnosis of adult ADHD: cultural influences and societal burden. J Atten Disord 16(5, Suppl)20S–38S. - PubMed
-
- Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, James LP, Wilson JT, Kearns GL, Leeder JS. (2007) Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther 81:510–516. - PubMed
-
- Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. (2012) Trends of outpatient prescription drug utilization in US children, 2002-2010. Pediatrics 130:23–31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

