Molecular Insights into the Impact of Oxidative Stress on the Quorum-Sensing Regulator Protein LasR
- PMID: 27053110
- PMCID: PMC4882445
- DOI: 10.1074/jbc.M116.719351
Molecular Insights into the Impact of Oxidative Stress on the Quorum-Sensing Regulator Protein LasR
Abstract
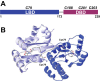
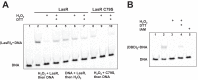
The LasR regulator protein functions at the top of the Pseudomonas aeruginosa quorum-sensing hierarchy and is implicated in promoting bacterial virulence. Of note is recent evidence that this transcription factor may also respond to oxidative stress. Here, all cysteines in LasR were inspected to deduce their redox sensitivity and to probe the connection between stress response and LasR activity using purified LasR and individual LasR domains. Cys(79) in the ligand binding domain of LasR appears to be important for ligand recognition and folding of this domain to potentiate DNA binding but does not seem to be sensitive to oxidative stress when bound to its native ligand. Two cysteines in the DNA binding domain of LasR do form a disulfide bond when treated with hydrogen peroxide, and formation of this Cys(201)-Cys(203) disulfide bond appears to disrupt the DNA binding activity of the transcription factor. Mutagenesis of either of these cysteines leads to expression of a protein that no longer binds DNA. A cell-based reporter assay linking LasR function with β-galactosidase activity gave results consistent with those obtained with purified LasR. This work provides a possible mechanism for oxidative stress response by LasR and indicates that multiple cysteines within the protein may prove to be useful targets for disabling its activity.
Keywords: DNA binding protein; Pseudomonas aeruginosa; antibiotic resistance; bacterial pathogenesis; bacterial transcription; cysteine; ligand-binding protein; quorum sensing.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Lyczak J. B., Cannon C. L., and Pier G. B. (2000) Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2, 1051–1060 - PubMed
-
- Bjarnsholt T., Jensen P. Ø., Jakobsen T. H., Phipps R., Nielsen A. K., Rybtke M. T., Tolker-Nielsen T., Givskov M., Høiby N., Ciofu O., and Scandinavian Cystic Fibrosis Study Consortium (2010) Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE 5, e10115. - PMC - PubMed
-
- Waters C. M., and Bassler B. L. (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 - PubMed
-
- Schuster M., Sexton D. J., Diggle S. P., and Greenberg E. P. (2013) Acyl-homoserine lactone quorum sensing: from evolution to application. Annu. Rev. Microbiol. 67, 43–63 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

