Brain-Wide Maps of Synaptic Input to Cortical Interneurons
- PMID: 27053207
- PMCID: PMC4821911
- DOI: 10.1523/JNEUROSCI.3967-15.2016
Brain-Wide Maps of Synaptic Input to Cortical Interneurons
Abstract
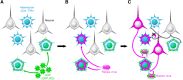
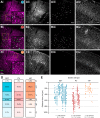
Cortical inhibition is mediated by diverse inhibitory neuron types that can each play distinct roles in information processing by virtue of differences in their input sources, intrinsic properties, and innervation targets. Previous studies in brain slices have demonstrated considerable cell-type specificity in laminar sources of local inputs. In contrast, little is known about possible differences in distant inputs to different cortical interneuron types. We used the monosynaptic rabies virus system, in conjunction with mice expressing Cre recombinase in either parvalbumin-positive, somatostatin-positive (SST+), or vasoactive intestinal peptide-positive (VIP+) neurons, to map the brain-wide input to the three major nonoverlapping classes of interneurons in mouse somatosensory cortex. We discovered that all three classes of interneurons received considerable input from known cortical and thalamic input sources, as well as from probable cholinergic cells in the basal nucleus of Meynert. Despite their common input sources, these classes differed in the proportion of long-distance cortical inputs originating from deep versus superficial layers. Similar to their laminar differences in local input, VIP+ neurons received inputs predominantly from deep layers while SST+ neurons received mostly superficial inputs. These classes also differed in the amount of input they received. Cortical and thalamic inputs were greatest onto VIP+ interneurons and smallest onto SST+ neurons.
Significance statement: These results indicate that all three major interneuron classes in the barrel cortex integrate both feedforward and feedback information from throughout the brain to modulate the activity of the local cortical circuit. However, differences in laminar sources and magnitude of distant cortical input suggest differential contributions from cortical areas. More input to vasoactive intestinal peptide-positive (VIP+) neurons than to somatostatin-positive (SST+) neurons suggests that disinhibition of the cortex via VIP+ cells, which inhibit SST+ cells, might be a general feature of long-distance corticocortical and thalamocortical circuits.
Keywords: S1; barrel cortex; cortex; inhibitory neuron.
Copyright © 2016 the authors 0270-6474/16/364000-10$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
