Counting Vesicular Release Events Reveals Binomial Release Statistics at Single Glutamatergic Synapses
- PMID: 27053208
- PMCID: PMC6705515
- DOI: 10.1523/JNEUROSCI.4352-15.2016
Counting Vesicular Release Events Reveals Binomial Release Statistics at Single Glutamatergic Synapses
Abstract
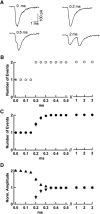
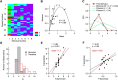
Many central glutamatergic synapses contain a single presynaptic active zone and a single postsynaptic density. However, the basic functional properties of such "simple synapses" remain unclear. One important step toward understanding simple synapse function is to analyze the number of synaptic vesicles released in such structures per action potential, but this goal has remained elusive until now. Here, we describe procedures that allow reliable vesicular release counting at simple synapses between parallel fibers and molecular layer interneurons of rat cerebellar slices. Our analysis involves local extracellular stimulation of single parallel fibers and deconvolution of resulting EPSCs using quantal signals as template. We observed a reduction of quantal amplitudes (amplitude occlusion) in pairs of consecutive EPSCs due to receptor saturation. This effect is larger (62%) than previously reported and primarily reflects receptor activation rather than desensitization. In addition to activation-driven amplitude occlusion, each EPSC reduces amplitudes of subsequent events by an estimated 3% due to cumulative desensitization. Vesicular release counts at simple synapses follow binomial statistics with a maximum that varies from 2 to 10 among experiments. This maximum presumably reflects the number of docking sites at a given synapse. These results show striking similarities, as well as significant quantitative differences, with respect to previous results at simple GABAergic synapses.
Significance statement: It is generally accepted that the output signal of individual central synapses saturates at high release probability, but it remains unclear whether the source of saturation is presynaptic, postsynaptic, or both presynaptic and postsynaptic. To clarify this and other issues concerning the function of synapses, we have developed new recording and analysis methods at single central glutamatergic synapses. We find that individual release events engage a high proportion of postsynaptic receptors (62%), revealing a larger component of postsynaptic saturation than anticipated. Conversely, we also find that the number of released synaptic vesicles is limited at each active zone. Altogether, our results argue for both presynaptic and postsynaptic contributions to signal saturation at single glutamatergic synapses.
Keywords: AMPA receptor; cerebellum; glutamate; synaptic release; synaptic vesicles.
Copyright © 2016 the authors 0270-6474/16/364010-16$15.00/0.
Figures







Similar articles
-
Prolonged physiological entrapment of glutamate in the synaptic cleft of cerebellar unipolar brush cells.J Neurophysiol. 1997 Sep;78(3):1320-33. doi: 10.1152/jn.1997.78.3.1320. J Neurophysiol. 1997. PMID: 9310423
-
Vesicular glutamate filling and AMPA receptor occupancy at the calyx of Held synapse of immature rats.J Physiol. 2009 May 15;587(Pt 10):2327-39. doi: 10.1113/jphysiol.2008.167759. Epub 2009 Mar 30. J Physiol. 2009. PMID: 19332485 Free PMC article.
-
Properties of transmission at a giant glutamatergic synapse in cerebellum: the mossy fiber-unipolar brush cell synapse.J Neurophysiol. 1995 Jul;74(1):24-42. doi: 10.1152/jn.1995.74.1.24. J Neurophysiol. 1995. PMID: 7472327
-
Quantal Fluctuations in Central Mammalian Synapses: Functional Role of Vesicular Docking Sites.Physiol Rev. 2017 Oct 1;97(4):1403-1430. doi: 10.1152/physrev.00032.2016. Physiol Rev. 2017. PMID: 28835509 Review.
-
Shaping excitation at glutamatergic synapses.Trends Neurosci. 1999 Oct;22(10):451-8. doi: 10.1016/s0166-2236(99)01445-9. Trends Neurosci. 1999. PMID: 10481192 Review.
Cited by
-
The functional properties of synapses made by regenerated axons across spinal cord lesion sites in lamprey.Neural Regen Res. 2022 Oct;17(10):2272-2277. doi: 10.4103/1673-5374.335828. Neural Regen Res. 2022. PMID: 35259849 Free PMC article.
-
Spatiotemporal dynamics of multi-vesicular release is determined by heterogeneity of release sites within central synapses.Elife. 2020 Feb 6;9:e55210. doi: 10.7554/eLife.55210. Elife. 2020. PMID: 32026806 Free PMC article.
-
Estimates of quantal synaptic parameters in light of more complex vesicle pool models.Front Cell Neurosci. 2025 Mar 18;19:1556360. doi: 10.3389/fncel.2025.1556360. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40170993 Free PMC article.
-
Inferring synaptic transmission from the stochastic dynamics of the quantal content: An analytical approach.PLoS Comput Biol. 2025 May 13;21(5):e1013067. doi: 10.1371/journal.pcbi.1013067. eCollection 2025 May. PLoS Comput Biol. 2025. PMID: 40359429 Free PMC article.
-
Approaches and Limitations in the Investigation of Synaptic Transmission and Plasticity.Front Synaptic Neurosci. 2019 Jul 24;11:20. doi: 10.3389/fnsyn.2019.00020. eCollection 2019. Front Synaptic Neurosci. 2019. PMID: 31396073 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
