Engineering Periodic shRNA for Enhanced Silencing Efficacy
- PMID: 27053374
- PMCID: PMC4923333
- DOI: 10.1038/mt.2016.69
Engineering Periodic shRNA for Enhanced Silencing Efficacy
Abstract
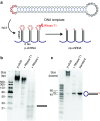
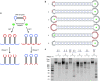
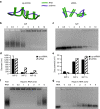
RNA interference (RNAi) provides a versatile therapeutic approach via silencing of specific genes, particularly undruggable targets in cancer and other diseases. However, challenges in the delivery of small interfering RNA (siRNA) have hampered clinical translation. Polymeric or periodic short hairpin RNAs (p-shRNAs)-synthesized by enzymatic amplification of circular DNA-are a recent development that can potentially address these delivery barriers by showing improved stability and complexation to enable nanoparticle packaging. Here, we modify these biomacromolecules via structural and sequence engineering coupled with selective enzymatic digestion to generate an open-ended p-shRNA (op-shRNA) that is cleaved over ten times more efficiently to yield siRNA. The op-shRNA induces considerably greater gene silencing than p-shRNA in multiple cancer cell lines up to 9 days. Furthermore, its high valency and flexibility dramatically improve complexation with a low molecular weight polycation compared to monomeric siRNA. Thus, op-shRNA provides an RNAi platform that can potentially be packaged and efficiently delivered to disease sites with higher therapeutic efficacy.
Figures


References
-
- Mok, H, Lee, SH, Park, JW and Park, TG (2010). Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nat Mater 9: 272–278. - PubMed
-
- Lee, SJ, Huh, MS, Lee, SY, Min, S, Lee, S, Koo, H et al. (2012). Tumor-homing poly-siRNA/glycol chitosan self-cross-linked nanoparticles for systemic siRNA delivery in cancer treatment. Angew Chem Int Ed Engl 51: 7203–7207. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

