Deficiency of the TLR4 analogue RP105 aggravates vein graft disease by inducing a pro-inflammatory response
- PMID: 27053419
- PMCID: PMC4823661
- DOI: 10.1038/srep24248
Deficiency of the TLR4 analogue RP105 aggravates vein graft disease by inducing a pro-inflammatory response
Abstract
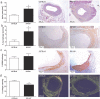
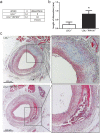
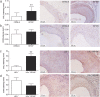
Venous grafts are often used to bypass occlusive atherosclerotic lesions; however, poor patency leads to vein graft disease. Deficiency of TLR4, an inflammatory regulator, reduces vein graft disease. Here, we investigate the effects of the accessory molecule and TLR4 analogue RadioProtective 105 (RP105) on vein graft disease. RP105 deficiency resulted in a 90% increase in vein graft lesion area compared to controls. In a hypercholesterolemic setting (LDLr(-/-)/RP105(-/-) versus LDLr(-/-) mice), which is of importance as vein graft disease is usually characterized by excessive atherosclerosis, total lesion area was not affected. However we did observe an increased number of unstable lesions and intraplaque hemorrhage upon RP105 deficiency. In both setups, lesional macrophage content, and lesional CCL2 was increased. In vitro, RP105(-/-) smooth muscle cells and mast cells secreted higher levels of CCL2. In conclusion, aggravated vein graft disease caused by RP105 deficiency results from an increased local inflammatory response.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Une D., Kulik A., Voisine P., Le May M. & Ruel M. Correlates of saphenous vein graft hyperplasia and occlusion 1 year after coronary artery bypass grafting: analysis from the CASCADE randomized trial. Circulation 128, S213–8 (2013). - PubMed
-
- Donker J. M. W. et al.. Midterm results of autologous saphenous vein and ePTFE pre-cuffed bypass surgery in peripheral arterial occlusive disease. Vasc Endovascular Surg 45, 598–603 (2011). - PubMed
-
- Fitzgibbon G. M. et al.. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 28, 616–26 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

