Radium-223 in Heavily Pretreated Metastatic Castrate-Resistant Prostate Cancer
- PMID: 27053499
- PMCID: PMC6849387
- DOI: 10.1016/j.clgc.2016.03.002
Radium-223 in Heavily Pretreated Metastatic Castrate-Resistant Prostate Cancer
Abstract
Background: Radium-223 is a bone-targeting radiopharmaceutical that extends survival in mCRPC. Postapproval data are limited, and the value of biochemical and radiologic monitoring during radium therapy is unknown.
Patients and methods: We conducted a retrospective study of 29 patients with mCRPC who received radium-223 at 1 of 3 participating institutions between August 2013 and December 2014. Trend of PSA, radiographic changes, and association of biochemical and clinical variables with PSA trend were measured.
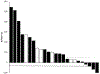
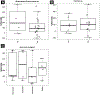
Results: The median age of patients was 70 years, 79% of patients (N = 23) were European Americans, and 17% of patients (N = 5) were African Americans. Twenty patients (69%) had received at least 3 lines of prior therapies. Some 38% of patients (N = 11) received all 6 cycles of radium-223. Twenty patients (69%) had an increase in PSA during radium therapy, and 4 patients (14%) had a decline in PSA levels. Five patients had visceral metastases on computed tomography imaging performed during the course of radium-223.
Conclusions: Radium therapy in mCRPC was associated with an increase in PSA in the majority of these heavily pretreated patients. The development of visceral disease was not uncommon, suggesting a need for follow-up computed tomography monitoring during radium-223 therapy. The significance of early increases in PSA and pain with radium-223 is still uncertain. Although pain and PSA flare have been reported in patients who subsequently have a dramatic response to therapy, we observed that a PSA increase or pain flare correlates to an improvement in bone scans only in a minority of patients.
Keywords: Alpha-emitter; Bone scan; Bone-targeting radiopharmaceutical; Pain flare-up; Prostate-specific antigen.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure
The authors have stated that they have no conflicts of interest.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351:1502–12. - PubMed
-
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004; 350:1655–64. - PubMed
-
- Lipton A. Implications of bone metastases and the benefits of bone-targeted therapy. Semin Oncol 2010; 37(Suppl 2):S15–29. - PubMed
-
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369:213–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

