Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis
- PMID: 27053504
- PMCID: PMC4912364
- DOI: 10.1634/theoncologist.2015-0440
Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis
Abstract
Background: Platinum-based neoadjuvant chemotherapy has been shown to improve survival outcomes in muscle-invasive bladder cancer patients. We performed a systematic review and meta-analysis to provide updated results of previous findings. We also summarized published data to compare clinical outcomes of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) versus gemcitabine and cisplatin/carboplatin (GC) in the neoadjuvant setting.
Methods: A meta-analysis of 15 randomized clinical trials was performed to compare neoadjuvant chemotherapy plus local treatment with the same local treatment alone. Because no randomized trials have investigated MVAC versus GC in the neoadjuvant setting, a meta-analysis of 13 retrospective studies was performed to compare MVAC with GC.
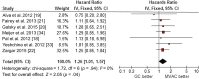
Results: A total of 3,285 patients were included in 15 randomized clinical trials. There was a significant overall survival (OS) benefit associated with cisplatin-based neoadjuvant chemotherapy (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.79-0.96). A total of 1,766 patients were included in 13 retrospective studies. There was no significant difference in pathological complete response between MVAC and GC. However, GC was associated with a significantly reduced overall survival (HR, 1.26; 95% CI, 1.01-1.57). After excluding carboplatin data, GC still seemed to be inferior to MVAC in OS (HR, 1.31; 95% CI, 0.99-1.74), but the difference was no longer statistically significant.
Conclusion: These results support the use of cisplatin-based combination neoadjuvant chemotherapy in muscle-invasive bladder cancer. Although GC and MVAC had similar treatment response rates, the different survival outcome observed in this study requires further investigation.
Implications for practice: Platinum-based neoadjuvant chemotherapy (NCT) has been shown to improve survival outcomes in muscle-invasive bladder cancer (MIBC) patients, but the optimal neoadjuvant regimen has not been established. Methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) and gemcitabine and cisplatin/carboplatin (GC) are two of the most commonly used chemotherapy regimens in modern oncology. In this two-step meta-analysis, an updated and more precise estimate of the survival benefit of cisplatin-based NCT in MIBC is provided. This study also demonstrated that MVAC might have superior overall survival compared with GC (with or without carboplatin data) in the neoadjuvant setting. The findings suggest that NCT should be standard care in MIBC, and MVAC could be the preferred neoadjuvant regimen.
Keywords: Bladder cancer; Chemotherapy; Neoadjuvant; Platinum; Survival.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures


Comment in
-
Systemic Therapy for Invasive Bladder Cancer: The Value Proposition.Oncologist. 2016 Jun;21(6):659-61. doi: 10.1634/theoncologist.2016-0074. Epub 2016 Apr 28. Oncologist. 2016. PMID: 27125752 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Chavan S, Bray F, Lortet-Tieulent J, et al. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59–73. - PubMed
-
- Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. - PubMed
-
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial J Clin Oncol 2011292171–2177. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

