Life Cycle Characterization of Sulfolobus Monocaudavirus 1, an Extremophilic Spindle-Shaped Virus with Extracellular Tail Development
- PMID: 27053548
- PMCID: PMC4886783
- DOI: 10.1128/JVI.00075-16
Life Cycle Characterization of Sulfolobus Monocaudavirus 1, an Extremophilic Spindle-Shaped Virus with Extracellular Tail Development
Abstract
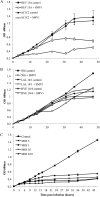
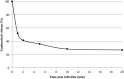
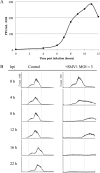
We provide here, for the first time, insights into the initial infection stages of a large spindle-shaped archaeal virus and explore the following life cycle events. Our observations suggest that Sulfolobus monocaudavirus 1 (SMV1) exhibits a high adsorption rate and that virions adsorb to the host cells via three distinct attachment modes: nosecone association, body association, and body/tail association. In the body/tail association mode, the entire virion, including the tail(s), aligns to the host cell surface and the main body is greatly flattened, suggesting a possible fusion entry mechanism. Upon infection, the intracellular replication cycle lasts about 8 h, at which point the virions are released as spindle-shaped tailless particles. Replication of the virus retarded host growth but did not cause lysis of the host cells. Once released from the host and at temperatures resembling that of its natural habitat, SMV1 starts developing one or two tails. This exceptional property of undergoing a major morphological development outside, and independently of, the host cell has been reported only once before for the related Acidianus two-tailed virus. Here, we show that SMV1 can develop tails of more than 900 nm in length, more than quadrupling the total virion length.
Importance: Very little is known about the initial life cycle stages of viruses infecting hosts of the third domain of life, Archaea This work describes the first example of an archaeal virus employing three distinct association modes. The virus under study, Sulfolobus monocaudavirus 1, is a representative of the large spindle-shaped viruses that are frequently found in acidic hot springs. The results described here will add valuable knowledge about Archaea, the least studied domain in the virology field.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Menzel P, Gudbergsdottir SR, Rike AG, Lin L, Zhang Q, Contursi P, Moracci M, Kristjansson JK, Bolduc B, Gavrilov S, Ravin N, Mardanov A, Bonch-Osmolovskaya E, Young M, Krogh A, Peng X. 2015. Comparative metagenomics of eight geographically remote terrestrial hot springs. Microb Ecol 70:411–424. doi:10.1007/s00248-015-0576-9. - DOI - PubMed
-
- Narasingarao P, Podell S, Ugalde JA, Brochier-Armanet C, Emerson JB, Brocks JJ, Heidelberg KB, Banfield JF, Allen EE. 2012. De novo metagenomic assembly reveals abundant novel major lineage of Archaea in hypersaline microbial communities. ISME J 6:81–93. doi:10.1038/ismej.2011.78. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

