Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer
- PMID: 27053631
- PMCID: PMC5530491
- DOI: 10.1136/gutjnl-2015-310977
Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer
Abstract
Objective: Colorectal cancer (CRC) is a major health concern. Vitamin D deficiency is associated with high CRC incidence and mortality, suggesting a protective effect of vitamin D against this disease. Given the strong influence of tumour stroma on cancer progression, we investigated the potential effects of the active vitamin D metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) on CRC stroma.
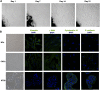
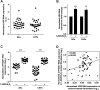
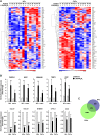
Design: Expression of vitamin D receptor (VDR) and two 1,25(OH)2D3 target genes was analysed in 658 patients with CRC with prolonged clinical follow-up. 1,25(OH)2D3 effects on primary cultures of patient-derived colon normal fibroblasts (NFs) and cancer-associated fibroblasts (CAFs) were studied using collagen gel contraction and migration assays and global gene expression analyses. Publicly available data sets (n=877) were used to correlate the 1,25(OH)2D3-associated gene signature in CAFs with CRC outcome.
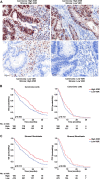
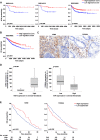
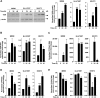
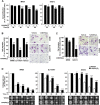
Results: High VDR expression in tumour stromal fibroblasts was associated with better overall survival (OS) and progression-free survival in CRC, independently of its expression in carcinoma cells. 1,25(OH)2D3 inhibited the protumoural activation of NFs and CAFs and imposed in CAFs a 1,25(OH)2D3-associated gene signature that correlated with longer OS and disease-free survival in CRC. Furthermore, expression of two genes from the signature, CD82 and S100A4, correlated with stromal VDR expression and clinical outcome in our cohort of patients with CRC.
Conclusions: 1,25(OH)2D3 has protective effects against CRC through the regulation of stromal fibroblasts. Accordingly, expression of VDR and 1,25(OH)2D3-associated gene signature in stromal fibroblasts predicts a favourable clinical outcome in CRC. Therefore, treatment of patients with CRC with VDR agonists could be explored even in the absence of VDR expression in carcinoma cells.
Keywords: COLORECTAL CANCER; MYOFIBROBLASTS; VITAMIN D RECEPTOR GENE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures

Comment in
-
VDR signaling inhibits cancer-associated-fibroblasts' release of exosomal miR-10a-5p and limits their supportive effects on pancreatic cancer cells.Gut. 2019 May;68(5):950-951. doi: 10.1136/gutjnl-2018-316627. Epub 2018 Apr 25. Gut. 2019. PMID: 29695492 No abstract available.
References
-
- International Agency For Research on Cancer (IARC). Vitamin D and cancer. IARC Working Group Reports Volume 5 Lyon, France: IARC, 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
