Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish
- PMID: 27053646
- PMCID: PMC4935499
- DOI: 10.1152/ajpregu.00250.2015
Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish
Abstract
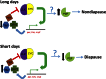
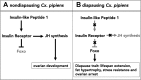
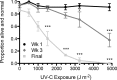
Life cycle delays are beneficial for opportunistic species encountering suboptimal environments. Many animals display a programmed arrest of development (diapause) at some stage(s) of their development, and the diapause state may or may not be associated with some degree of metabolic depression. In this review, we will evaluate current advancements in our understanding of the mechanisms responsible for the remarkable phenotype, as well as environmental cues that signal entry and termination of the state. The developmental stage at which diapause occurs dictates and constrains the mechanisms governing diapause. Considerable progress has been made in clarifying proximal mechanisms of metabolic arrest and the signaling pathways like insulin/Foxo that control gene expression patterns. Overlapping themes are also seen in mechanisms that control cell cycle arrest. Evidence is emerging for epigenetic contributions to diapause regulation via small RNAs in nematodes, crustaceans, insects, and fish. Knockdown of circadian clock genes in selected insect species supports the importance of clock genes in the photoperiodic response that cues diapause. A large suite of chaperone-like proteins, expressed during diapause, protects biological structures during long periods of energy-limited stasis. More information is needed to paint a complete picture of how environmental cues are coupled to the signal transduction that initiates the complex diapause phenotype, as well as molecular explanations for how the state is terminated. Excellent examples of molecular memory in post-dauer animals have been documented in Caenorhabditis elegans It is clear that a single suite of mechanisms does not regulate diapause across all species and developmental stages.
Keywords: cell cycle; development; diapause; dormancy; metabolism.
Copyright © 2016 the American Physiological Society.
Figures






References
-
- Altiero T, Guidetti R, Caselli V, Cesari M, Rebecchi L. Ultraviolet radiation tolerance in hydrated and desiccated eutardigrades. J Zool System Evol Res 49: 104–110, 2011.
-
- Anchordoguy TJ, Hand SC. Acute blockage of the ubiquitin-mediated proteolytic pathway during invertebrate quiescence. Am J Physiol Regul Integr Comp Physiol 267: R895–R900, 1994. - PubMed
-
- Anchordoguy TJ, Hand SC. Reactivation of ubiquitination in Artemia franciscana embryos during recovery from anoxia-induced quiescence. J Exp Biol 198: 1299–1305, 1995. - PubMed
-
- Anchordoguy TJ, Hofmann G, Hand SC. Extension of enzyme half-life during quiescence in Artemia embryos. Am J Physiol Regul Integr Comp Physiol 264: R85–R89, 1993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

