The beneficial role of proteolysis in skeletal muscle growth and stress adaptation
- PMID: 27054028
- PMCID: PMC4822268
- DOI: 10.1186/s13395-016-0086-6
The beneficial role of proteolysis in skeletal muscle growth and stress adaptation
Erratum in
-
Erratum to: The beneficial role of proteolysis in skeletal muscle growth and stress adaptation.Skelet Muscle. 2016 May 4;6:19. doi: 10.1186/s13395-016-0090-x. eCollection 2016. Skelet Muscle. 2016. PMID: 27148436 Free PMC article.
Abstract
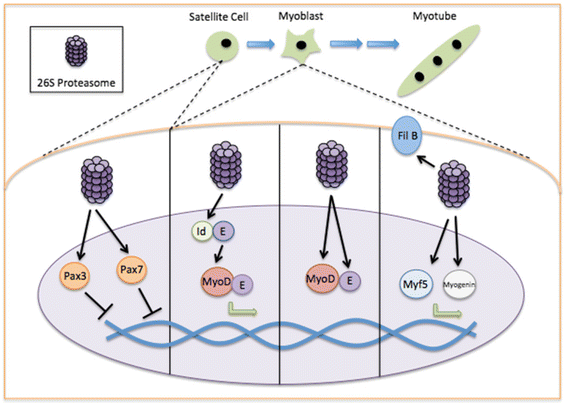
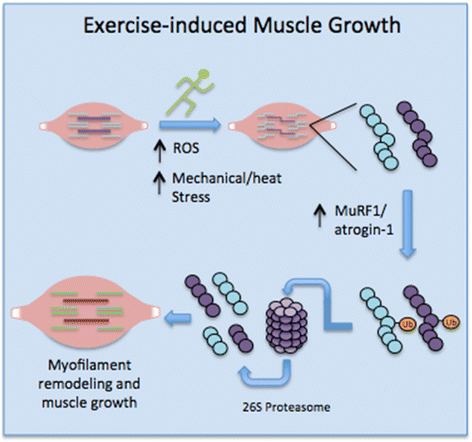
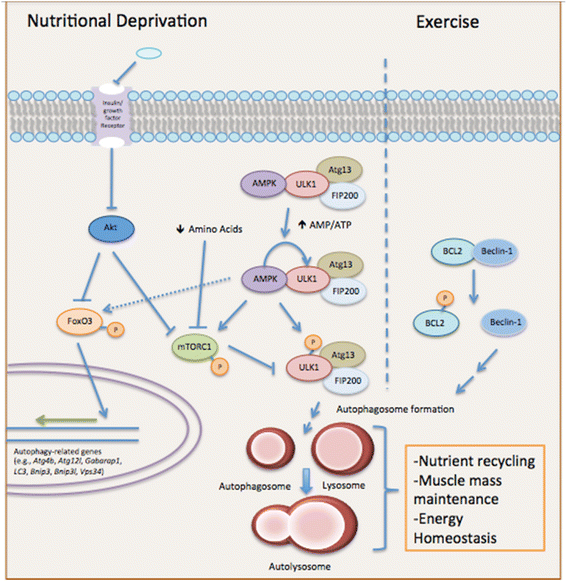
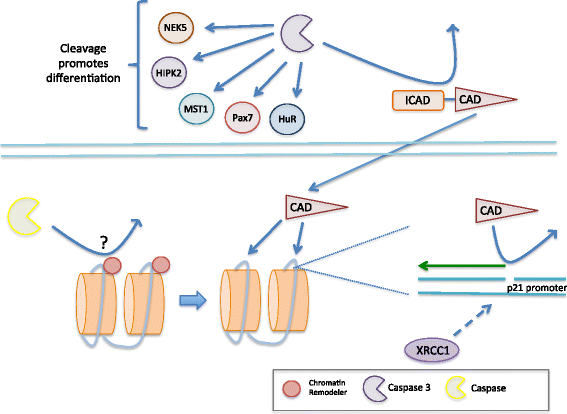
Muscle atrophy derived from excessive proteolysis is a hallmark of numerous disease conditions. Accordingly, the negative consequences of skeletal muscle protein breakdown often overshadow the critical nature of proteolytic systems in maintaining normal cellular function. Here, we discuss the major cellular proteolysis machinery-the ubiquitin/proteosome system, the autophagy/lysosomal system, and caspase-mediated protein cleavage-and the critical role of these protein machines in establishing and preserving muscle health. We examine how ordered degradation modifies (1) the spatiotemporal expression of myogenic regulatory factors during myoblast differentiation, (2) membrane fusion during myotube formation, (3) sarcomere remodeling and muscle growth following physical stress, and (4) energy homeostasis during nutrient deprivation. Finally, we review the origin and etiology of a number of myopathies and how these devastating conditions arise from inborn errors in proteolysis.
Keywords: Autophagy; Caspase; Muscle cell differentiation; Muscle growth; Proteasome; Proteolysis.
Figures
References
-
- Abu Hatoum O, Gross-Mesilaty S, Breitschopf K, Hoffman A, Gonen H, Ciechanover A, et al. Degradation of myogenic transcription factor MyoD by the ubiquitin pathway in vivo and in vitro: regulation by specific DNA binding. Mol Cell Biol. 1998;18:5670–5677. doi: 10.1128/MCB.18.10.5670. - DOI - PMC - PubMed
-
- Augusto V, Padovani CR, Campos GER. Skeletal muscle fibre types in C57BL6J mice. Braz J Morphol Sci. 2004;21:89–94.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

