Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum microRNAs
- PMID: 27054342
- PMCID: PMC4824492
- DOI: 10.1371/journal.pone.0153200
Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum microRNAs
Abstract
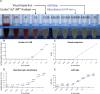
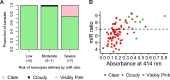
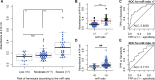
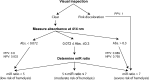
microRNAs have emerged as powerful regulators of many biological processes, and their expression in many cancer tissues has been shown to correlate with clinical parameters such as cancer type and prognosis. Present in a variety of biological fluids, microRNAs have been described as a 'gold mine' of potential noninvasive biomarkers. Release of microRNA content of blood cells upon hemolysis dramatically alters the microRNA profile in blood, potentially affecting levels of a significant number of proposed biomarker microRNAs and, consequently, accuracy of serum or plasma-based tests. Several methods to detect low levels of hemolysis have been proposed; however, a direct comparison assessing their sensitivities is currently lacking. In this study, we evaluated the sensitivities of four methods to detect hemolysis in serum (listed in the order of sensitivity): measurement of hemoglobin using a Coulter® AcT diff™ Analyzer, visual inspection, the absorbance of hemoglobin measured by spectrophotometry at 414 nm and the ratio of red blood cell-enriched miR-451a to the reference microRNA miR-23a-3p. The miR ratio detected hemolysis down to approximately 0.001%, whereas the Coulter® AcT diff™ Analyzer was unable to detect hemolysis lower than 1%. The spectrophotometric method could detect down to 0.004% hemolysis, and correlated with the miR ratio. Analysis of hemolysis in a cohort of 86 serum samples from cancer patients and healthy controls showed that 31 of 86 (36%) were predicted by the miR ratio to be hemolyzed, whereas only 8 of these samples (9%) showed visible pink discoloration. Using receiver operator characteristic (ROC) analyses, we identified absorbance cutoffs of 0.072 and 0.3 that could identify samples with low and high levels of hemolysis, respectively. Overall, this study will assist researchers in the selection of appropriate methodologies to test for hemolysis in serum samples prior to quantifying expression of microRNAs.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

