External Quality Assessment for Tuberculosis Diagnosis and Drug Resistance in the European Union: A Five Year Multicentre Implementation Study
- PMID: 27055064
- PMCID: PMC4824391
- DOI: 10.1371/journal.pone.0152926
External Quality Assessment for Tuberculosis Diagnosis and Drug Resistance in the European Union: A Five Year Multicentre Implementation Study
Abstract
Background: External quality assurance (EQA) systems are essential to ensure accurate diagnosis of TB and drug-resistant TB. The implementation of EQA through organising regular EQA rounds and identification of training needs is one of the key activities of the European TB reference laboratory network (ERLTB-Net). The aim of this study was to analyse the results of the EQA rounds in a systematic manner and to identify potential benefits as well as common problems encountered by the participants.
Methods: The ERLTB-Net developed seven EQA modules to test laboratories' proficiency for TB detection and drug susceptibility testing using both conventional and rapid molecular tools. All National TB Reference laboratories in the European Union and European Economic Area (EU/EEA) Member States were invited to participate in the EQA scheme.
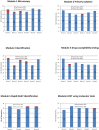
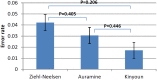
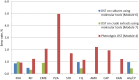
Results: A total of 32 National TB Reference laboratories participated in six EQA rounds conducted in 2010-2014. The participation rate ranged from 52.9% - 94.1% over different modules and rounds. Overall, laboratories demonstrated very good proficiency proving their ability to diagnose TB and drug-resistant TB with high accuracy in a timely manner. A small number of laboratories encountered problems with identification of specific Non-tuberculous Mycobacteria (NTMs) (N = 5) and drug susceptibility testing to Pyrazinamide, Amikacin, Capreomycin, and Ethambutol (N = 4).
Conclusions: The European TB Reference laboratories showed a steady and high level of performance in the six EQA rounds. A network such as ERLTB-Net can be instrumental in developing and implementing EQA and in establishing collaboration between laboratories to improve the diagnosis of TB in the EU/EEA.
Conflict of interest statement
Figures

References
-
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2015. Stockholm: European Centre for Disease Prevention and Control, 2015.
-
- Drobniewski FA, Nikolayevskyy V, Hoffner S, Pogoryelova O, Manissero D, et al. (2008) The added value of a European Union tuberculosis reference laboratory network—analysis of the national reference laboratory activities. Euro Surveill 13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

