B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus
- PMID: 27055816
- PMCID: PMC4823887
- DOI: 10.1186/s12974-016-0537-3
B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus
Abstract
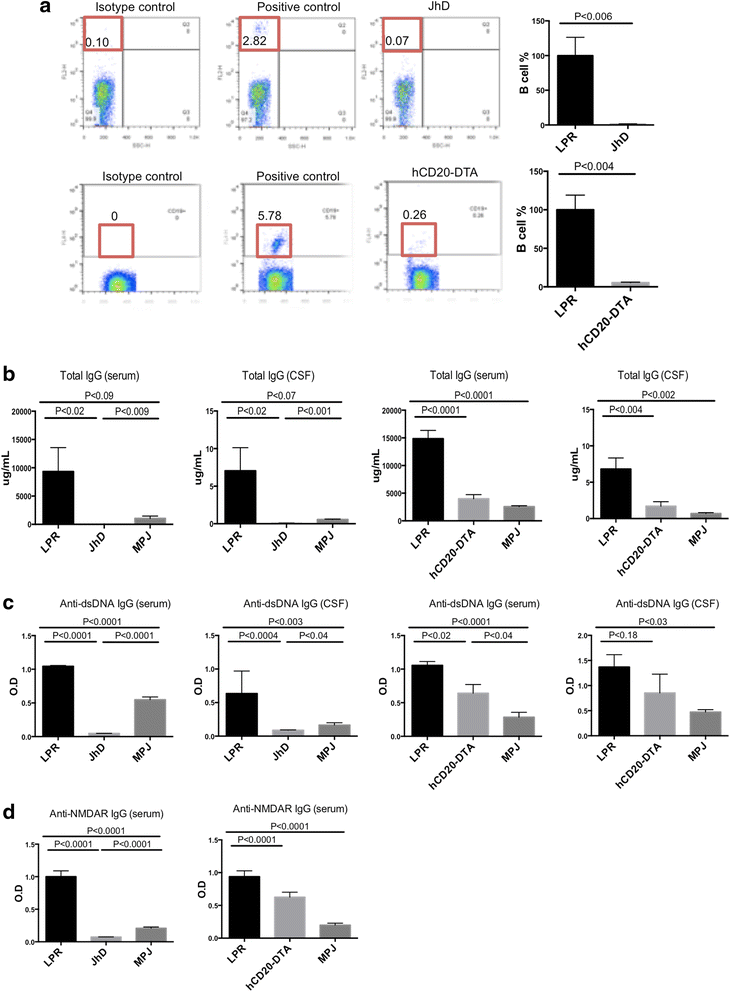
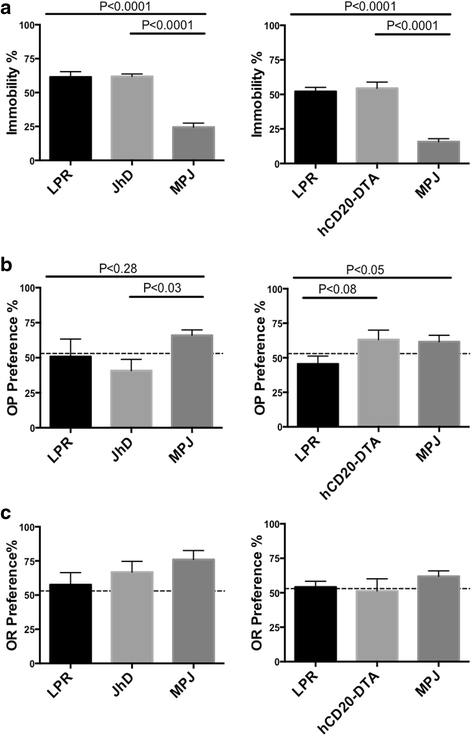
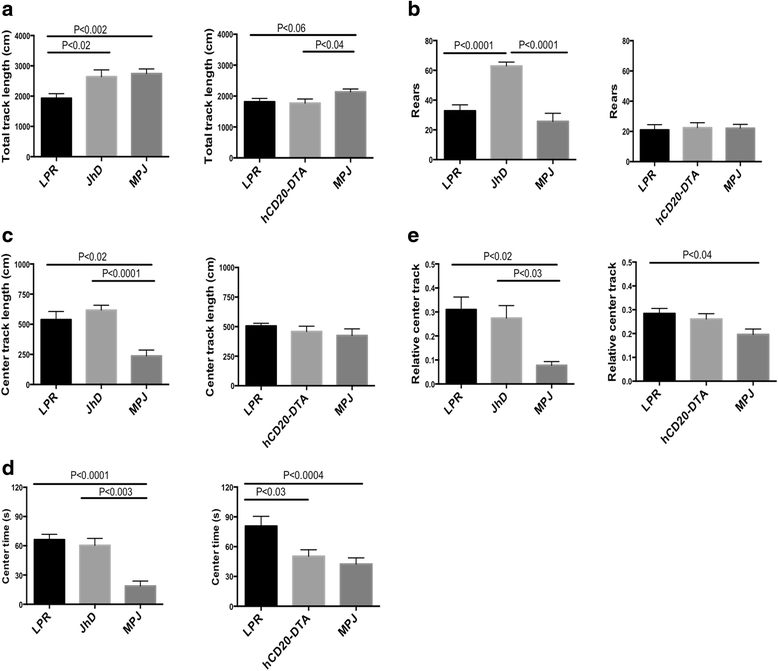
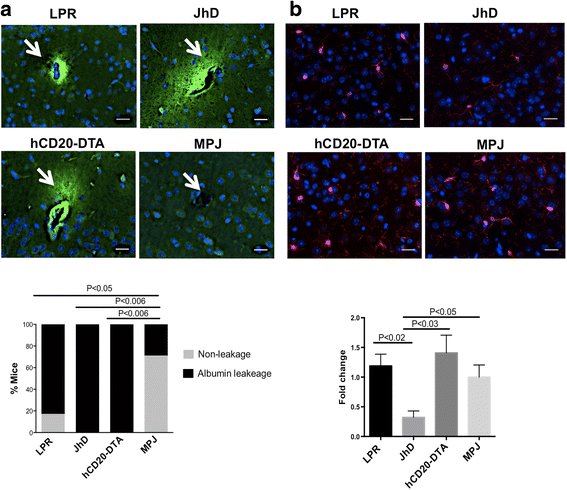
Background: Neuropsychiatric lupus (NPSLE) can be one of the earliest clinical manifestations in human lupus. However, its mechanisms are not fully understood. In lupus, a compromised blood-brain barrier may allow for the passage of circulating autoantibodies into the brain, where they can induce neuropsychiatric abnormalities including depression-like behavior and cognitive abnormalities. The purpose of this study was to determine the role of B cells and/or autoantibodies in the pathogenesis of murine NPSLE.
Methods: We evaluated neuropsychiatric manifestations, brain pathology, and cytokine expression in constitutively (JhD/MRL/lpr) and conditionally (hCD20-DTA/MRL/lpr, inducible by tamoxifen) B cell-depleted mice as compared to MRL/lpr lupus mice.
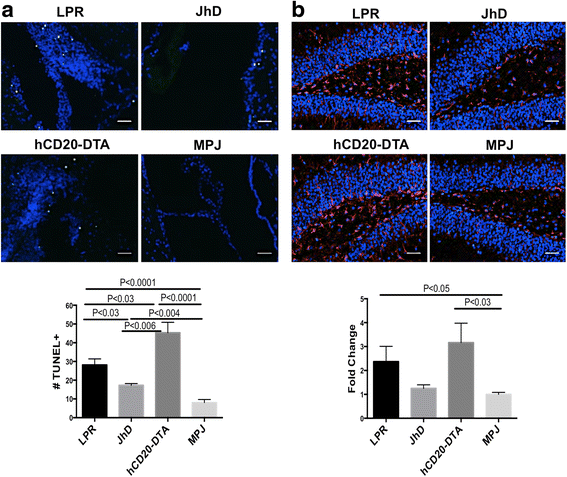
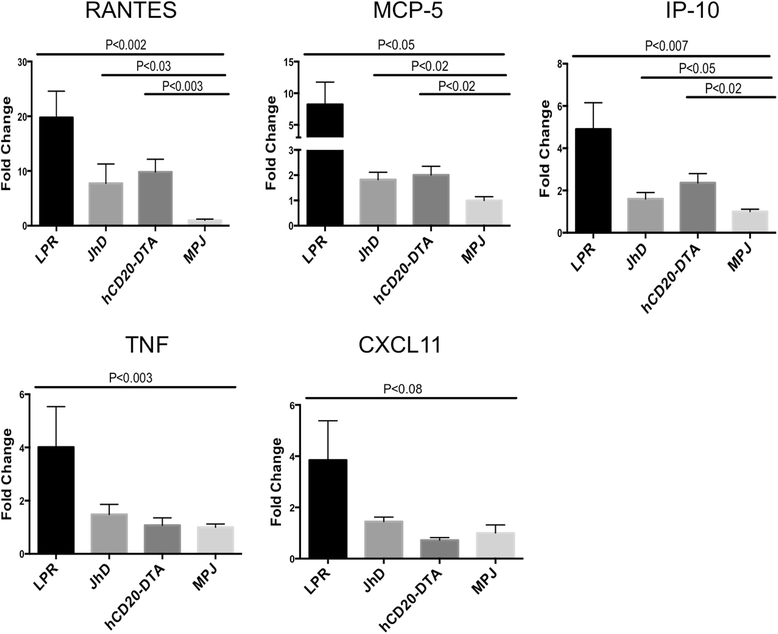
Results: We found that autoantibody levels were negligible (JhD/MRL/lpr) or significantly reduced (hCD20-DTA/MRL/lpr) in the serum and cerebrospinal fluid, respectively. Nevertheless, both JhD/MRL/lpr and hCD20-DTA/MRL/lpr mice showed profound depression-like behavior, which was no different from MRL/lpr mice. Cognitive deficits were also observed in both JhD/MRL/lpr and hCD20-DTA/MRL/lpr mice, similar to those exhibited by MRL/lpr mice. Furthermore, although some differences were dependent on the timing of depletion, central features of NPSLE in the MRL/lpr strain including increased blood-brain barrier permeability, brain cell apoptosis, and upregulated cytokine expression persisted in B cell-deficient and B cell-depleted mice.
Conclusions: Our study surprisingly found that B cells and/or autoantibodies are not required for key features of neuropsychiatric disease in murine NPSLE.
Keywords: Autoantibodies; B cells; Lupus; Neuropsychiatric SLE; SLE.
Figures
Similar articles
-
Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway.J Autoimmun. 2013 Jun;43:44-54. doi: 10.1016/j.jaut.2013.03.002. Epub 2013 Apr 8. J Autoimmun. 2013. PMID: 23578591 Free PMC article.
-
Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice.J Neuroinflammation. 2015 Nov 6;12:205. doi: 10.1186/s12974-015-0423-4. J Neuroinflammation. 2015. PMID: 26546449 Free PMC article.
-
Neuropsychiatric Systemic Lupus Erythematosus Is Dependent on Sphingosine-1-Phosphate Signaling.Front Immunol. 2018 Sep 26;9:2189. doi: 10.3389/fimmu.2018.02189. eCollection 2018. Front Immunol. 2018. PMID: 30319641 Free PMC article.
-
The central and multiple roles of B cells in lupus pathogenesis.Immunol Rev. 1999 Jun;169:107-21. doi: 10.1111/j.1600-065x.1999.tb01310.x. Immunol Rev. 1999. PMID: 10450512 Review.
-
Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model.Autoimmun Rev. 2014 Sep;13(9):963-73. doi: 10.1016/j.autrev.2014.08.015. Epub 2014 Aug 23. Autoimmun Rev. 2014. PMID: 25183233 Review.
Cited by
-
CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus.Clin Immunol. 2017 Dec;185:100-108. doi: 10.1016/j.clim.2016.08.019. Epub 2016 Aug 26. Clin Immunol. 2017. PMID: 27570219 Free PMC article.
-
All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class.Mol Psychiatry. 2017 Dec;22(12):1776-1784. doi: 10.1038/mp.2016.125. Epub 2016 Aug 9. Mol Psychiatry. 2017. PMID: 27502473
-
TWEAKing the Hippocampus: The Effects of TWEAK on the Genomic Fabric of the Hippocampus in a Neuropsychiatric Lupus Mouse Model.Genes (Basel). 2021 Jul 29;12(8):1172. doi: 10.3390/genes12081172. Genes (Basel). 2021. PMID: 34440346 Free PMC article.
-
Mechanisms of neuropsychiatric lupus: The relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier.J Autoimmun. 2018 Jul;91:34-44. doi: 10.1016/j.jaut.2018.03.001. Epub 2018 Apr 4. J Autoimmun. 2018. PMID: 29627289 Free PMC article.
-
Organ-based characterization of B cells in patients with systemic lupus erythematosus.Front Immunol. 2025 Jan 23;16:1509033. doi: 10.3389/fimmu.2025.1509033. eCollection 2025. Front Immunol. 2025. PMID: 39917309 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

