Familial lecithin:cholesterol acyltransferase deficiency: First-in-human treatment with enzyme replacement
- PMID: 27055967
- PMCID: PMC4826469
- DOI: 10.1016/j.jacl.2015.12.007
Familial lecithin:cholesterol acyltransferase deficiency: First-in-human treatment with enzyme replacement
Abstract
Background: Humans with familial lecithin:cholesterol acyltransferase (LCAT) deficiency (FLD) have extremely low or undetectable high-density lipoprotein cholesterol (HDL-C) levels and by early adulthood develop many manifestations of the disorder, including corneal opacities, anemia, and renal disease.
Objective: To determine if infusions of recombinant human LCAT (rhLCAT) could reverse the anemia, halt progression of renal disease, and normalize HDL in FLD.
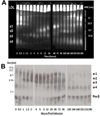
Methods: rhLCAT (ACP-501) was infused intravenously over 1 hour on 3 occasions in a dose optimization phase (0.3, 3.0, and 9.0 mg/kg), then 3.0 or 9.0 mg/kg every 1 to 2 weeks for 7 months in a maintenance phase. Plasma lipoproteins, lipids, LCAT levels, and several measures of renal function and other clinical labs were monitored.
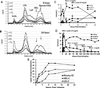
Results: LCAT concentration peaked at the end of each infusion and decreased to near baseline over 7 days. Renal function generally stabilized or improved and the anemia improved. After infusion, HDL-C rapidly increased, peaking near normal in 8 to 12 hours; analysis of HDL particles by various methods all revealed rapid sequential disappearance of preβ-HDL and small α-4 HDL and appearance of normal α-HDL. Low-density lipoprotein cholesterol increased more slowly than HDL-C. Of note, triglyceride routinely decreased after meals after infusion, in contrast to the usual postprandial increase in the absence of rhLCAT infusion.
Conclusions: rhLCAT infusions were well tolerated in this first-in-human study in FLD; the anemia improved, as did most parameters related to renal function in spite of advanced disease. Plasma lipids transiently normalized, and there was rapid sequential conversion of small preβ-HDL particles to mature spherical α-HDL particles.
Keywords: Cholesterol; HDL; LCAT; Lecithin cholesterol acyltransferase; Lecithin cholesterol acyltransferase deficiency; Lipoprotein-X; Recombinant enzyme replacement; Renal disease; Triglyceride.
Published by Elsevier Inc.
Figures





References
-
- Santamarina-Fojo S, Hoeg JM, Assmann G, Brewer HB. Lecithin Cholesterol Acyltransferase Deficiency and Fish Eye Disease. In: Scriver CR, Beaudet D, Valle D, Sly WS, editors. The Metabolic & Molecular Basis of Inherited Disease. New York, NY: McGraw-Hill; 2001. pp. 2817–2833.
-
- Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. Journal of Lipid Research. 2004;45:1594–1607. - PubMed
-
- Barter PJ. Hugh sinclair lecture: the regulation and remodelling of HDL by plasma factors. Atherosclerosis Supplements. 2002;3:39–47. - PubMed
-
- Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. Journal of Lipid Research. 1968;9:155–167. - PubMed
-
- Norum K, Gjone E, Glomset JA. Familial Lecithin:cholesterol Acyltransferase Deficiency, Including Fish Eye Disease. In: Scriver CR, Beaudet AL, Sly WS, editors. The Metabolic Basis of Inherited Disease. New York, NY: McGraw-Hill; 1989. pp. 1181–1194.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

