Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition
- PMID: 27056296
- PMCID: PMC5042670
- DOI: 10.1681/ASN.2015091055
Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition
Abstract
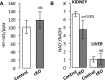
The circadian clock controls a wide variety of metabolic and homeostatic processes in a number of tissues, including the kidney. However, the role of the renal circadian clocks remains largely unknown. To address this question, we performed a combined functional, transcriptomic, and metabolomic analysis in mice with inducible conditional knockout (cKO) of BMAL1, which is critically involved in the circadian clock system, in renal tubular cells (Bmal1lox/lox/Pax8-rtTA/LC1 mice). Induction of cKO in adult mice did not produce obvious abnormalities in renal sodium, potassium, or water handling. Deep sequencing of the renal transcriptome revealed significant changes in the expression of genes related to metabolic pathways and organic anion transport in cKO mice compared with control littermates. Furthermore, kidneys from cKO mice exhibited a significant decrease in the NAD+-to-NADH ratio, which reflects the oxidative phosphorylation-to-glycolysis ratio and/or the status of mitochondrial function. Metabolome profiling showed significant changes in plasma levels of amino acids, biogenic amines, acylcarnitines, and lipids. In-depth analysis of two selected pathways revealed a significant increase in plasma urea level correlating with increased renal Arginase II activity, hyperargininemia, and increased kidney arginine content as well as a significant increase in plasma creatinine concentration and a reduced capacity of the kidney to secrete anionic drugs (furosemide) paralleled by an approximate 80% decrease in the expression level of organic anion transporter 3 (SLC22a8). Collectively, these results indicate that the renal circadian clocks control a variety of metabolic/homeostatic processes at the intrarenal and systemic levels and are involved in drug disposition.
Keywords: arginase; circadian clock; kidney excretory rhythms; metabolome; organic anion transporter.
Copyright © 2016 by the American Society of Nephrology.
Figures



Comment in
-
Circadian rhythms: Functional roles of the renal tubular circadian clock.Nat Rev Nephrol. 2016 Jun;12(6):316. doi: 10.1038/nrneph.2016.60. Epub 2016 Apr 25. Nat Rev Nephrol. 2016. PMID: 27108840 No abstract available.
References
-
- Bollinger T, Schibler U: Circadian rhythms - from genes to physiology and disease. Swiss Med Wkly 144: w13984, 2014 - PubMed
-
- Firsov D, Bonny O: Circadian regulation of renal function. Kidney Int 78: 640–645, 2010 - PubMed
-
- Bonny O, Firsov D: Circadian regulation of renal function and potential role in hypertension. Curr Opin Nephrol Hypertens 22: 439–444, 2013 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

