Distinct Membrane Disruption Pathways Are Induced by 40-Residue β-Amyloid Peptides
- PMID: 27056326
- PMCID: PMC4933272
- DOI: 10.1074/jbc.M116.720656
Distinct Membrane Disruption Pathways Are Induced by 40-Residue β-Amyloid Peptides
Abstract
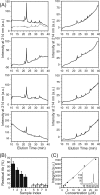
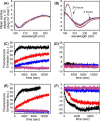
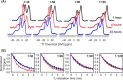
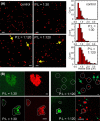
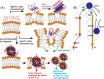
Cellular membrane disruption induced by β-amyloid (Aβ) peptides has been considered one of the major pathological mechanisms for Alzheimer disease. Mechanistic studies of the membrane disruption process at a high-resolution level, on the other hand, are hindered by the co-existence of multiple possible pathways, even in simplified model systems such as the phospholipid liposome. Therefore, separation of these pathways is crucial to achieve an in-depth understanding of the Aβ-induced membrane disruption process. This study, which utilized a combination of multiple biophysical techniques, shows that the peptide-to-lipid (P:L) molar ratio is an important factor that regulates the selection of dominant membrane disruption pathways in the presence of 40-residue Aβ peptides in liposomes. Three distinct pathways (fibrillation with membrane content leakage, vesicle fusion, and lipid uptake through a temporarily stable ionic channel) become dominant in model liposome systems under specific conditions. These individual systems are characterized by both the initial states of Aβ peptides and the P:L molar ratio. Our results demonstrated the possibility to generate simplified Aβ-membrane model systems with a homogeneous membrane disruption pathway, which will benefit high-resolution mechanistic studies in the future. Fundamentally, the possibility of pathway selection controlled by P:L suggests that the driving forces for Aβ aggregation and Aβ-membrane interactions may be similar at the molecular level.
Keywords: Alzheimer disease; NMR; amyloid β; ion channel; liposome; membrane.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Chiti F., and Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 - PubMed
-
- Chiang P. K., Lam M. A., and Luo Y. (2008) The many faces of amyloid β in Alzheimer's disease. Curr. Mol. Med. 8, 580–584 - PubMed
-
- Eckert G. P., Wood W. G., and Müller W. E. (2010) Lipid membranes and β-amyloid: a harmful connection. Curr. Protein Pept. Sci. 11, 319–325 - PubMed
-
- Butterfield S. M., and Lashuel H. A. (2010) Amyloidogenic protein-membrane interactions: mechanistic insight from model systems. Angew. Chem. Int. Ed. Engl. 49, 5628–5654 - PubMed
-
- Mason R. P., Estermyer J. D., Kelly J. F., and Mason P. E. (1996) Alzheimer's disease amyloid β peptide 25–35 is localized in the membrane hydrophobic core: x-ray diffraction analysis. Biochem. Biophys. Res. Comm. 222, 78–82 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

