The insect central complex as model for heterochronic brain development-background, concepts, and tools
- PMID: 27056385
- PMCID: PMC4896989
- DOI: 10.1007/s00427-016-0542-7
The insect central complex as model for heterochronic brain development-background, concepts, and tools
Abstract
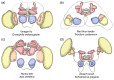
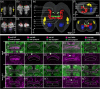
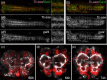
The adult insect brain is composed of neuropils present in most taxa. However, the relative size, shape, and developmental timing differ between species. This diversity of adult insect brain morphology has been extensively described while the genetic mechanisms of brain development are studied predominantly in Drosophila melanogaster. However, it has remained enigmatic what cellular and genetic mechanisms underlie the evolution of neuropil diversity or heterochronic development. In this perspective paper, we propose a novel approach to study these questions. We suggest using genome editing to mark homologous neural cells in the fly D. melanogaster, the beetle Tribolium castaneum, and the Mediterranean field cricket Gryllus bimaculatus to investigate developmental differences leading to brain diversification. One interesting aspect is the heterochrony observed in central complex development. Ancestrally, the central complex is formed during embryogenesis (as in Gryllus) but in Drosophila, it arises during late larval and metamorphic stages. In Tribolium, it forms partially during embryogenesis. Finally, we present tools for brain research in Tribolium including 3D reconstruction and immunohistochemistry data of first instar brains and the generation of transgenic brain imaging lines. Further, we characterize reporter lines labeling the mushroom bodies and reflecting the expression of the neuroblast marker gene Tc-asense, respectively.
Keywords: Brain; Central complex; Drosophila; Evolution; Heterochrony; Tribolium.
Figures

Similar articles
-
Metamorphosis and adult development of the mushroom bodies of the red flour beetle, Tribolium castaneum.Dev Neurobiol. 2008 Nov;68(13):1487-502. doi: 10.1002/dneu.20669. Dev Neurobiol. 2008. PMID: 18792069
-
Structure of the mushroom bodies of the insect brain.Annu Rev Entomol. 2006;51:209-32. doi: 10.1146/annurev.ento.51.110104.150954. Annu Rev Entomol. 2006. PMID: 16332210 Review.
-
Sequence heterochrony led to a gain of functionality in an immature stage of the central complex: A fly-beetle insight.PLoS Biol. 2020 Oct 26;18(10):e3000881. doi: 10.1371/journal.pbio.3000881. eCollection 2020 Oct. PLoS Biol. 2020. PMID: 33104689 Free PMC article.
-
The Red Flour Beetle as Model for Comparative Neural Development: Genome Editing to Mark Neural Cells in Tribolium Brain Development.Methods Mol Biol. 2020;2047:191-217. doi: 10.1007/978-1-4939-9732-9_11. Methods Mol Biol. 2020. PMID: 31552656
-
Structure and function of the homeotic gene complex (HOM-C) in the beetle, Tribolium castaneum.Bioessays. 1993 Jul;15(7):439-44. doi: 10.1002/bies.950150702. Bioessays. 1993. PMID: 11536538 Review.
Cited by
-
A key role for foxQ2 in anterior head and central brain patterning in insects.Development. 2017 Aug 15;144(16):2969-2981. doi: 10.1242/dev.147637. Epub 2017 Jul 25. Development. 2017. PMID: 28811313 Free PMC article.
-
Development of the Neurochemical Architecture of the Central Complex.Front Behav Neurosci. 2016 Aug 31;10:167. doi: 10.3389/fnbeh.2016.00167. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27630548 Free PMC article. Review.
-
The red flour beetle T. castaneum: elaborate genetic toolkit and unbiased large scale RNAi screening to study insect biology and evolution.Evodevo. 2022 Jul 19;13(1):14. doi: 10.1186/s13227-022-00201-9. Evodevo. 2022. PMID: 35854352 Free PMC article. Review.
-
Adult neurogenesis in the mushroom bodies of red flour beetles (Tribolium castaneum, HERBST) is influenced by the olfactory environment.Sci Rep. 2020 Jan 23;10(1):1090. doi: 10.1038/s41598-020-57639-x. Sci Rep. 2020. PMID: 31974446 Free PMC article.
-
Olfactory navigation in arthropods.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023 Jul;209(4):467-488. doi: 10.1007/s00359-022-01611-9. Epub 2023 Jan 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023. PMID: 36658447 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

