Dominant negative SNARE peptides stabilize the fusion pore in a narrow, release-unproductive state
- PMID: 27056575
- PMCID: PMC11108528
- DOI: 10.1007/s00018-016-2213-2
Dominant negative SNARE peptides stabilize the fusion pore in a narrow, release-unproductive state
Abstract
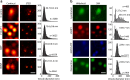
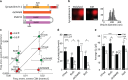
Key support for vesicle-based release of gliotransmitters comes from studies of transgenic mice with astrocyte-specific expression of a dominant-negative domain of synaptobrevin 2 protein (dnSNARE). To determine how this peptide affects exocytosis, we used super-resolution stimulated emission depletion microscopy and structured illumination microscopy to study the anatomy of single vesicles in astrocytes. Smaller vesicles contained amino acid and peptidergic transmitters and larger vesicles contained ATP. Discrete increases in membrane capacitance, indicating single-vesicle fusion, revealed that astrocyte stimulation increases the frequency of predominantly transient fusion events in smaller vesicles, whereas larger vesicles transitioned to full fusion. To determine whether this reflects a lower density of SNARE proteins in larger vesicles, we treated astrocytes with botulinum neurotoxins D and E, which reduced exocytotic events of both vesicle types. dnSNARE peptide stabilized the fusion-pore diameter to narrow, release-unproductive diameters in both vesicle types, regardless of vesicle diameter.
Keywords: Astrocytes; Capacitance measurements; Fusion pore; Gliotransmitters; Regulated exocytosis; STED microscopy.
Figures




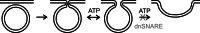
References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22(5):208–215. pii:S0166-2236(98)01349-6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

