Phase separation of signaling molecules promotes T cell receptor signal transduction
- PMID: 27056844
- PMCID: PMC4892427
- DOI: 10.1126/science.aad9964
Phase separation of signaling molecules promotes T cell receptor signal transduction
Abstract
Activation of various cell surface receptors triggers the reorganization of downstream signaling molecules into micrometer- or submicrometer-sized clusters. However, the functional consequences of such clustering have been unclear. We biochemically reconstituted a 12-component signaling pathway on model membranes, beginning with T cell receptor (TCR) activation and ending with actin assembly. When TCR phosphorylation was triggered, downstream signaling proteins spontaneously separated into liquid-like clusters that promoted signaling outputs both in vitro and in human Jurkat T cells. Reconstituted clusters were enriched in kinases but excluded phosphatases and enhanced actin filament assembly by recruiting and organizing actin regulators. These results demonstrate that protein phase separation can create a distinct physical and biochemical compartment that facilitates signaling.
Copyright © 2016, American Association for the Advancement of Science.
Figures


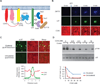
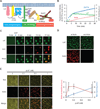
Comment in
-
CELL SIGNALING. Liquidity in immune cell signaling.Science. 2016 Apr 29;352(6285):516-7. doi: 10.1126/science.aaf8179. Science. 2016. PMID: 27126023 No abstract available.
References
-
- Bienz M. Signalosome assembly by domains undergoing dynamic head-to-tail polymerization. Trends in biochemical sciences. 2014;39:487–495. - PubMed
-
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. - PubMed
-
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

