Urinary APE1/Ref-1: A Potential Bladder Cancer Biomarker
- PMID: 27057081
- PMCID: PMC4745317
- DOI: 10.1155/2016/7276502
Urinary APE1/Ref-1: A Potential Bladder Cancer Biomarker
Abstract
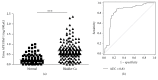
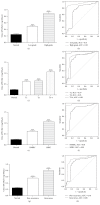
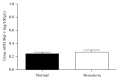
Bladder cancer (BCa) is one of the most common urothelial cancers with still noticeable incidence rate. Early detection of BCa is highly correlated with successful therapeutic outcomes. We previously showed that apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) was expressed at an increased level in the serum of BCa patients when compared to the level in healthy controls. In this study, we investigated whether urinary APE1/Ref-1 was also elevated in patients with BCa. In this case-control study, voided urine was collected from 277 subjects including 169 BCa patients and 108 non-BCa controls. Urinary APE1/Ref-1 level was assessed by enzyme-linked immunosorbent assay (ELISA). APE1/Ref-1 levels were significantly elevated in BCa patients relative to levels in non-BCa controls and were correlated with tumor grade and stage. Urinary APE1/Ref-1 levels were also higher in patients with recurrence history of BCa. The receiver operating characteristics (ROC) curve of APE1/Ref-1 showed an area under the curve of 0.83, indicating the reliability and validity of this biomarker. The optimal combination of sensitivity and specificity was determined to be 82% and 80% at a cut-off value of 0.376 ng/100 μL for detection of APE1/Ref-1 in urine. In conclusion, urinary APE1/Ref-1 levels measured from noninvasively obtained body fluids would be clinically applicable for diagnosis of BCa.
Figures
References
-
- Millán-Rodríguez F., Chéchile-Toniolo G., Salvador-Bayarri J., Palou J., Algaba F., Vicente-Rodríguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. Journal of Urology. 2000;164(3, part 1):680–684. doi: 10.1016/s0022-5347(05)67280-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

