Chinese Herbal Medicine Fuzheng Kang-Ai Decoction Inhibited Lung Cancer Cell Growth through AMPKα-Mediated Induction and Interplay of IGFBP1 and FOXO3a
- PMID: 27057199
- PMCID: PMC4757679
- DOI: 10.1155/2016/5060757
Chinese Herbal Medicine Fuzheng Kang-Ai Decoction Inhibited Lung Cancer Cell Growth through AMPKα-Mediated Induction and Interplay of IGFBP1 and FOXO3a
Abstract
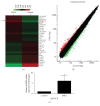
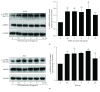
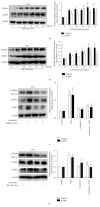
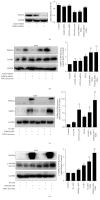
The aim of this study is to investigate the actions of Chinese herbal medicine, called "Fuzheng Kang-Ai" (FZKA for short) decoction, against non-small cell lung cancer (NSCLC) and its mechanisms in vitro and in vivo. We showed that the effect of FZKA decoction significantly inhibited growth of A549 and PC9 cells. Furthermore, FZKA increased phosphorylation of AMP-activated protein kinase alpha (AMPKα) and induced protein expression of insulin-like growth factor (IGF) binding protein 1 (IGFBP1) and forkhead homeobox type O3a (FOXO3a). The specific inhibitor of AMPKα (Compound C) blocked FZKA-induced protein expression of IGFBP1 and FOXO3a. Interestingly, silencing of IGFBP1 and FOXO3a overcame the inhibitory effect of FZKA on cell growth. Moreover, silencing of IGFBP1 attenuated the effect of FZKA decoction on FOXO3a expression, and exogenous expression of FOXO3a enhanced the FZKA-stimulated phosphorylation of AMPKα. Accordingly, FZKA inhibited the tumor growth in xenograft nude mice model. Collectively, our results show that FZKA decoction inhibits proliferation of NSCLC cells through activation of AMPKα, followed by induction of IGFBP1 and FOXO3a proteins. Exogenous expression of FOXO3a feedback enhances FZKA decoction-stimulated IGFBP1 expression and phosphorylation of AMPKα. The reciprocal interplay of IGFBP1 and FOXO3a contribute to the overall responses of FAKA decoction.
Figures




Similar articles
-
Fuzheng Kang-Ai decoction enhances the effect of Gefitinib-induced cell apoptosis in lung cancer through mitochondrial pathway.Cancer Cell Int. 2020 May 24;20:185. doi: 10.1186/s12935-020-01270-3. eCollection 2020. Cancer Cell Int. 2020. PMID: 32489321 Free PMC article.
-
Decoction of Chinese Herbal Medicine Fuzheng Kang-Ai Induces Lung Cancer Cell Apoptosis via STAT3/Bcl-2/Caspase-3 Pathway.Evid Based Complement Alternat Med. 2018 Jun 25;2018:8567905. doi: 10.1155/2018/8567905. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30046347 Free PMC article.
-
Chinese herbal medicine Fuzheng Kang-Ai decoction sensitized the effect of gefitinib on inhibition of human lung cancer cells through inactivating PI3-K/Akt -mediated suppressing MUC1 expression.J Ethnopharmacol. 2016 Dec 24;194:918-929. doi: 10.1016/j.jep.2016.10.077. Epub 2016 Oct 28. J Ethnopharmacol. 2016. PMID: 27989877
-
The repression and reciprocal interaction of DNA methyltransferase 1 and specificity protein 1 contributes to the inhibition of MET expression by the combination of Chinese herbal medicine FZKA decoction and erlotinib.J Ethnopharmacol. 2019 Jul 15;239:111928. doi: 10.1016/j.jep.2019.111928. Epub 2019 May 8. J Ethnopharmacol. 2019. PMID: 31077779
-
Emodin Increases Expression of Insulin-Like Growth Factor Binding Protein 1 through Activation of MEK/ERK/AMPKα and Interaction of PPARγ and Sp1 in Lung Cancer.Cell Physiol Biochem. 2017;41(1):339-357. doi: 10.1159/000456281. Epub 2017 Jan 26. Cell Physiol Biochem. 2017. PMID: 28214826
Cited by
-
Fuzheng Kang-Ai decoction enhances the effect of Gefitinib-induced cell apoptosis in lung cancer through mitochondrial pathway.Cancer Cell Int. 2020 May 24;20:185. doi: 10.1186/s12935-020-01270-3. eCollection 2020. Cancer Cell Int. 2020. PMID: 32489321 Free PMC article.
-
Decoction of Chinese Herbal Medicine Fuzheng Kang-Ai Induces Lung Cancer Cell Apoptosis via STAT3/Bcl-2/Caspase-3 Pathway.Evid Based Complement Alternat Med. 2018 Jun 25;2018:8567905. doi: 10.1155/2018/8567905. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30046347 Free PMC article.
-
GPX4 Plays a Crucial Role in Fuzheng Kang'ai Decoction-Induced Non-Small Cell Lung Cancer Cell Ferroptosis.Front Pharmacol. 2022 Apr 13;13:851680. doi: 10.3389/fphar.2022.851680. eCollection 2022. Front Pharmacol. 2022. PMID: 35496303 Free PMC article.
-
Gefitinib plus Fuzheng Kang'ai Formula () in Patients with Advanced Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Mutation: A Randomized Controlled Trial.Chin J Integr Med. 2018 Oct;24(10):734-740. doi: 10.1007/s11655-017-2819-8. Epub 2017 Aug 9. Chin J Integr Med. 2018. PMID: 28795387 Clinical Trial.
-
Traditional Chinese medicine, Fuzheng Kang‑Ai decoction, inhibits metastasis of lung cancer cells through the STAT3/MMP9 pathway.Mol Med Rep. 2017 Sep;16(3):2461-2468. doi: 10.3892/mmr.2017.6905. Epub 2017 Jun 30. Mol Med Rep. 2017. PMID: 28677797 Free PMC article.
References
-
- Malik P. S., Malik A., Deo S. V., Mohan A., Mohanti B. K., Raina V. Underutilization of curative treatment among patients with non small cell lung cancer: experience from a tertiary care centre in India. Asian Pacific Journal of Cancer Prevention. 2014;15(6):2875–2878. doi: 10.7314/apjcp.2014.15.6.2875. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

