Evaluation of hyperglycaemic response to intra-operative dexamethasone administration in patients undergoing elective intracranial surgery: A randomised, prospective study
- PMID: 27057213
- PMCID: PMC4802961
- DOI: 10.4103/1793-5482.177660
Evaluation of hyperglycaemic response to intra-operative dexamethasone administration in patients undergoing elective intracranial surgery: A randomised, prospective study
Abstract
Background and aim: The glucocorticoid dexamethasone in a bolus dose of 8-10 mg followed by quarterly dose of 4 mg is commonly used during intracranial surgery so as to reduce oedema and vascular permeability. However, the detrimental hyperglycaemic effects of dexamethasone may override its potentially beneficial effects. The present prospective, randomised study aimed at comparing the degree and magnitude of hyperglycaemia induced by prophylactic administration of dexamethasone in patients undergoing elective craniotomy.
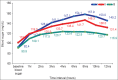
Materials and methods: Sixty American Society of Anaesthesiologist (ASA) grade-I and II patients were randomly assigned to three groups of 20 patients each. Group-I received dexamethasone during surgery for the first time. Group-II received dexamethasone in addition to receiving it pre-operatively, whereas Group-III (control group) patients were administered normal saline as placebo. Baseline blood glucose (BG) was measured in all the three groups before induction of anaesthesia and thereafter after every hour for 4 h and then two-hourly. Besides intra- and intergroup comparison of BG, peak BG concentration was also recorded for each patient. Statistical analysis was carried out with analysis of variance (ANOVA) and Student's t-test and value of P < 0.05 was considered statistically significant.
Results: Baseline BG reading were higher and statistically significant in Group-II as compared with Group-I and Group-III (P < 0.05). However, peak BG levels were significantly higher in Group-I than in Group-II and III (P < 0.05). Similarly, the magnitude of change in peak BG was significantly higher in Group-I as compared to Group-II and III (P < 0.05).
Conclusion: Peri-operative administration of dexamethasone during neurosurgical procedures can cause significant increase in BG concentration especially in patients who receive dexamethasone intra-operatively only.
Keywords: Dexamethasone; hyperglycaemic response; intracranial surgery.
Conflict of interest statement
Figures
References
-
- Galicich JH, French LA. Use of dexamethasone in the treatment of cerebral edema resulting from brain tumors and brain surgery. Am J Pract Dig Treat. 1961;12:169–74. - PubMed
-
- Mclelland S, Long DM. Genesis of the use of corticosteroids in the treatment and prevention of brain edema. Neurosurgery. 2008;62:965–8. - PubMed
-
- Hockey B, Leslie K, Williams D. Dexamethasone for intracranial neurosurgery and anesthesia. J Clin Neurosci. 2009;16:1389–93. - PubMed
-
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth. 2006;97:164–70. - PubMed
-
- Leenders KL, Beaney RP, Brooks DJ, Lammertsma AA, Heather JD, McKenzie CG. Dexamethasone treatment of brain tumor patients. Effects on regional cerebral blood flow, blood volume and oxygen utilization. Neurology. 1985;36:1400–3. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources