Inhibition of Receptor Interacting Protein Kinases Attenuates Cardiomyocyte Hypertrophy Induced by Palmitic Acid
- PMID: 27057269
- PMCID: PMC4736315
- DOI: 10.1155/2016/1451676
Inhibition of Receptor Interacting Protein Kinases Attenuates Cardiomyocyte Hypertrophy Induced by Palmitic Acid
Abstract
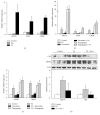
Palmitic acid (PA) is known to cause cardiomyocyte dysfunction. Cardiac hypertrophy is one of the important pathological features of PA-induced lipotoxicity, but the mechanism by which PA induces cardiomyocyte hypertrophy is still unclear. Therefore, our study was to test whether necroptosis, a receptor interacting protein kinase 1 and 3 (RIPK1 and RIPK3-) dependent programmed necrosis, was involved in the PA-induced cardiomyocyte hypertrophy. We used the PA-treated primary neonatal rat cardiac myocytes (NCMs) or H9c2 cells to study lipotoxicity. Our results demonstrated that cardiomyocyte hypertrophy was induced by PA treatment, determined by upregulation of hypertrophic marker genes and cell surface area enlargement. Upon PA treatment, the expression of RIPK1 and RIPK3 was increased. Pretreatment with the RIPK1 inhibitor necrostatin-1 (Nec-1), the PA-induced cardiomyocyte hypertrophy, was attenuated. Knockdown of RIPK1 or RIPK3 by siRNA suppressed the PA-induced myocardial hypertrophy. Moreover, a crosstalk between necroptosis and endoplasmic reticulum (ER) stress was observed in PA-treated cardiomyocytes. Inhibition of RIPK1 with Nec-1, phosphorylation level of AKT (Ser473), and mTOR (Ser2481) was significantly reduced in PA-treated cardiomyocytes. In conclusion, RIPKs-dependent necroptosis might be crucial in PA-induced myocardial hypertrophy. Activation of mTOR may mediate the effect of necroptosis in cardiomyocyte hypertrophy induced by PA.
Figures



Similar articles
-
RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis.FEBS J. 2014 Nov;281(21):4921-34. doi: 10.1111/febs.13034. Epub 2014 Oct 4. FEBS J. 2014. PMID: 25195660
-
RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death.Apoptosis. 2017 May;22(5):626-638. doi: 10.1007/s10495-017-1358-2. Apoptosis. 2017. PMID: 28289909
-
Akt and mTOR mediate programmed necrosis in neurons.Cell Death Dis. 2014 Feb 27;5(2):e1084. doi: 10.1038/cddis.2014.69. Cell Death Dis. 2014. PMID: 24577082 Free PMC article.
-
The regulation of necroptosis and perspectives for the development of new drugs preventing ischemic/reperfusion of cardiac injury.Apoptosis. 2022 Oct;27(9-10):697-719. doi: 10.1007/s10495-022-01760-x. Epub 2022 Aug 20. Apoptosis. 2022. PMID: 35986803 Review.
-
Receptor Interacting Protein Kinases 1/3: The Potential Therapeutic Target for Cardiovascular Inflammatory Diseases.Front Pharmacol. 2021 Nov 18;12:762334. doi: 10.3389/fphar.2021.762334. eCollection 2021. Front Pharmacol. 2021. PMID: 34867386 Free PMC article. Review.
Cited by
-
Mitochondrial function in the heart: the insight into mechanisms and therapeutic potentials.Br J Pharmacol. 2019 Nov;176(22):4302-4318. doi: 10.1111/bph.14431. Epub 2018 Aug 2. Br J Pharmacol. 2019. PMID: 29968316 Free PMC article. Review.
-
Inhibition of P2X7 Purinergic Receptor Ameliorates Cardiac Fibrosis by Suppressing NLRP3/IL-1β Pathway.Oxid Med Cell Longev. 2020 May 21;2020:7956274. doi: 10.1155/2020/7956274. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32566102 Free PMC article.
-
Implications of Necroptosis for Cardiovascular Diseases.Curr Med Sci. 2019 Aug;39(4):513-522. doi: 10.1007/s11596-019-2067-6. Epub 2019 Jul 25. Curr Med Sci. 2019. PMID: 31346984 Review.
-
Inhibition of Mitofusin-2 Promotes Cardiac Fibroblast Activation via the PERK/ATF4 Pathway and Reactive Oxygen Species.Oxid Med Cell Longev. 2019 Apr 16;2019:3649808. doi: 10.1155/2019/3649808. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31178957 Free PMC article.
-
Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury.Cell Mol Life Sci. 2021 Jan;78(1):63-78. doi: 10.1007/s00018-020-03587-8. Epub 2020 Jun 28. Cell Mol Life Sci. 2021. PMID: 32596778 Free PMC article. Review.
References
-
- Xu Q., Chen S. Y., Deng L. D., Feng L. P., Huang L. Z., Yu R. R. Antioxidant effect of mogrosides against oxidative stress induced by palmitic acid in mouse insulinoma NIT-1 cells. Brazilian Journal of Medical and Biological Research. 2013;46(11):949–955. doi: 10.1590/1414-431X20133163. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

